Copper catalyst for selective hydrogenation of mixed C4 and preparation method of copper catalyst
A technology for selective hydrogenation and copper catalysts, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
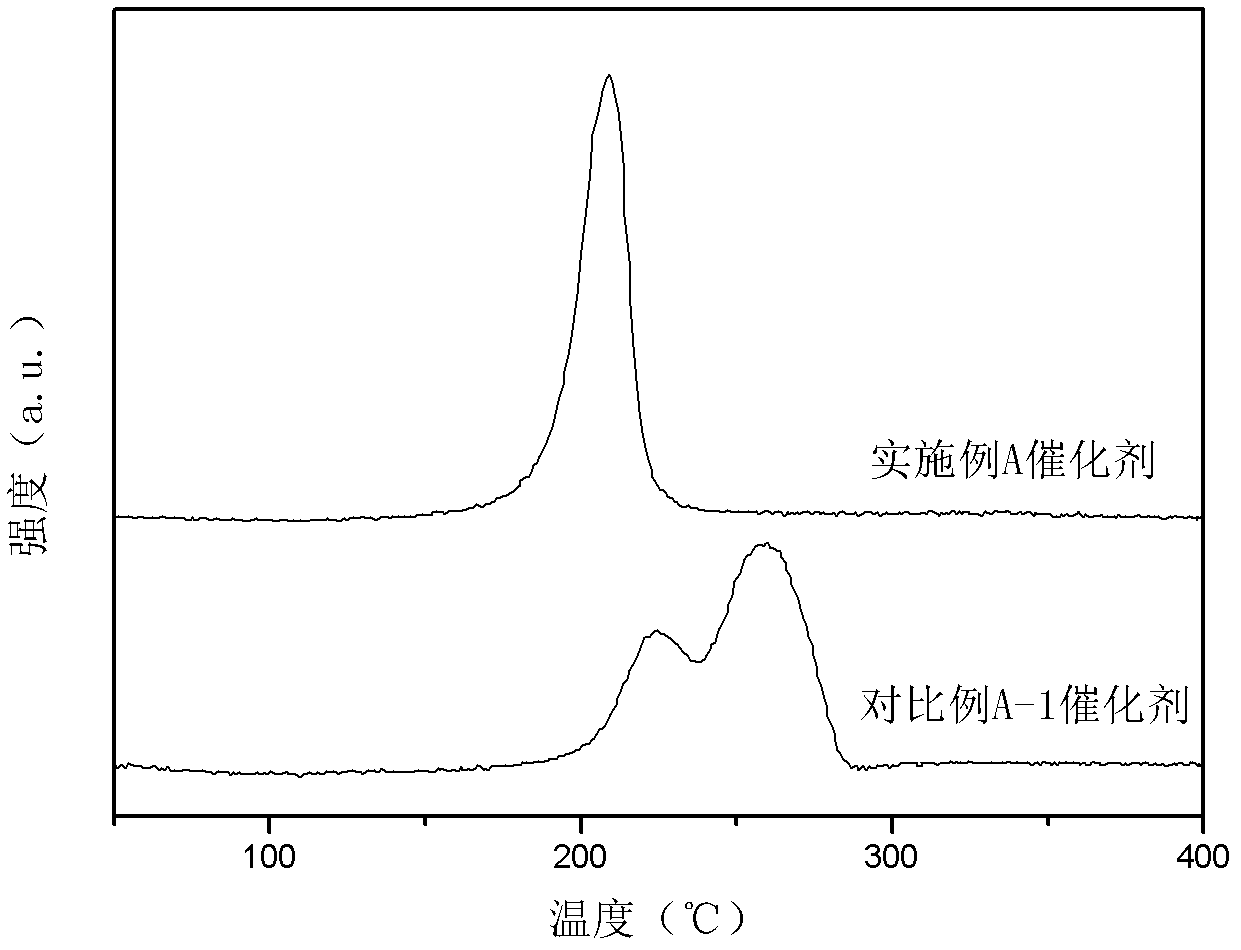
Image
Examples
Embodiment 1
[0020] Weigh 38g of Cu(NO 3 ) 2· 3H 2 O, 0.79g of AgNO 3 and 9.9g of Co(NO 3 ) 2· 6H 2 O, measure butylamine 38.2mL, make the ratio of butylamine and Cu, Ag, Co total mole number be 2: 1, butylamine and 30mL water are mixed uniformly, then Cu(NO 3 ) 2· 3H 2 O, AgNO 3 and Co(NO 3 ) 2· 6H 2 O is added to the aqueous solution of butylamine to dissolve evenly, and it is made into an impregnating solution.
[0021] Weigh 87.5gAl 2 o 3 Tooth ball carrier, its specific surface is 197m 2 / g, the pore volume is 0.6mL / g. Spray the above impregnating solution on the Al 2 o 3 Carrier, then aged for 5 hours, dried at 120°C for 10 hours, and calcined at 400°C for 4 hours to finally obtain Catalyst A. The content of Cu in catalyst A is 10wt%, the content of Ag is 0.5wt%, and the content of Co is 2wt%.
Embodiment 2
[0026] Weigh 38g of Cu(NO 3 ) 2· 3H 2 O, 9.9g of Ni(NO 3 ) 2· 6H 2 O, 4.6g 50% Mn(NO 3 ) 2 solution, 3.8gCr(NO 3 ) 3 9H 2 O, 0.32g of AgNO 3 and 4.9g of Co(NO 3 ) 2· 6H 2 O. Measure 21.9 mL of diethanolamine, so that the ratio of diethanolamine to the total moles of Cu, Ni, Mn, Cr, Ag, Co is 1:1, mix diethanolamine and 35 mL of water evenly, and then mix Cu(NO 3 ) 2· 3H 2 O, Ni(NO 3 ) 2· 6H 2 O, Mn(NO 3 ) 2 , Cr(NO 3 ) 3 9H 2 O, AgNO 3 , Co(NO 3 ) 2· 6H 2 O is dissolved in an aqueous solution of diethanolamine to prepare an impregnating solution.
[0027] Weigh 85.8gAl 2 o 3 Small ball carrier, its specific surface is 115m 2 / g, the pore volume is 0.5mL / g. Spray the above impregnating solution on the Al 2 o 3 Carrier, then aged for 5 hours, dried at 120°C for 10 hours, and calcined at 400°C for 4 hours to finally obtain Catalyst B. Cu content is 10wt% in the catalyst B, and Ni content is 2wt%, and Mn content is 0.5wt%, and Cr content is 0.5wt...
Embodiment 3
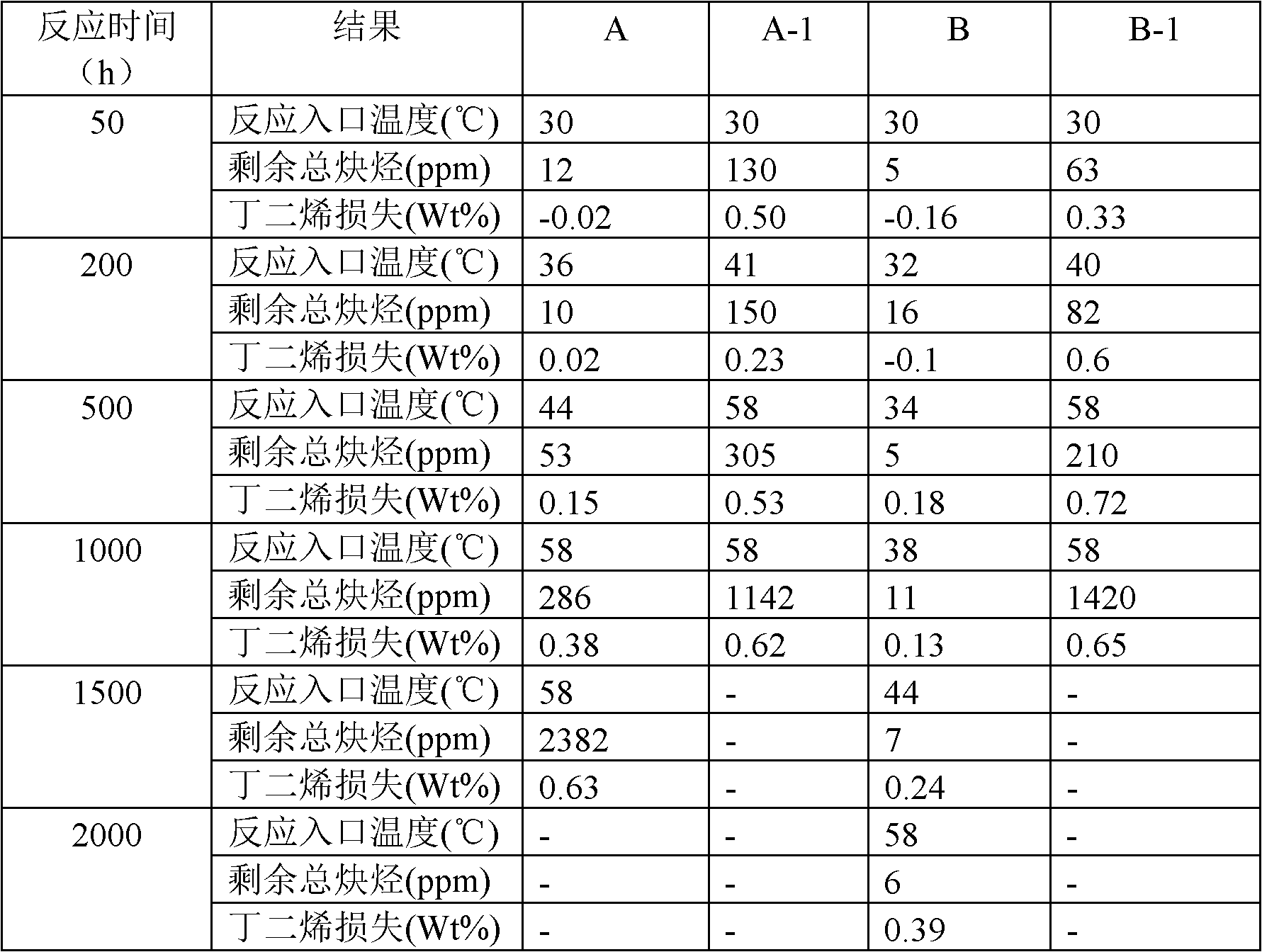
[0032] The present embodiment evaluates the catalyst of the present invention, and the test conditions are as follows:
[0033] The catalyst test was carried out in a fixed bed reactor with a catalyst loading of 200mL. Before the reaction, it must be replaced with nitrogen and then reduced with hydrogen at 250°C for 4 hours. The composition of the mixed C4 raw materials is listed in Table 1. Flow through the reactor from bottom to top, the catalyst bed inlet temperature is 30-60°C, the reaction pressure is 1.0MPa, and the liquid space velocity of the C4 fraction is 4h -1 , The molar ratio of hydrogen to alkyne (often referred to as hydrogen-alkyne ratio) is 2-4.
[0034] Calculation Method of Residual Alkyne and Butadiene Loss in Reaction
[0035] 1. Calculation of remaining total alkynes:
[0036] Total alkynes remaining = VA remaining in reaction + EA remaining in reaction + MA remaining in reaction
[0037] 2. Calculation of butadiene loss:
[0038] Butadiene loss wt% =...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com