Synthesis method of silicon-based photochromic elastomer containing naphthol pyran with cross-linked structure
A photochromic and naphthol pyran technology, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, etc., can solve the problems such as no silicon-based photochromic elastomers, and achieve rich varieties, excellent performance and simple operation. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
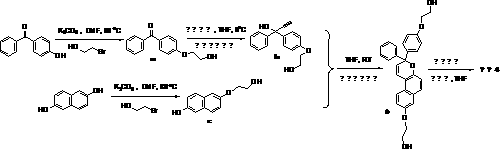
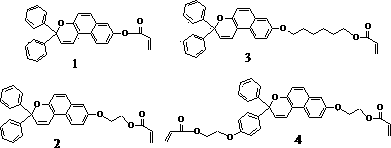
[0040] Example 1: In a 100ml three-necked flask equipped with a reflux condenser and a dropping funnel, add 1.5g of hydrogen-containing silicone oil, 25ml of toluene and 0.004g of chloroplatinic acid in water, and heat it after feeding argon for 15 minutes. When the temperature rises to 65 o At C, add 0.3g monomer dropwise 1 and 0.1g monomer 4 15ml of the toluene mixed solution was dropped within 2 hours, and then continued to be heated to reflux, and the reaction was stopped after 2 hours. Distill under reduced pressure to remove the remaining toluene, and dissolve the residue in a small amount of tetrahydrofuran solvent, remove the catalyst through an aluminum oxide column, and settle in cold methanol for 3 times to obtain a photochromic elastomer. The product is a solid substance with very low hardness , 1.64g, yield 86.3%. The structural formula of the product is as follows:
[0041]
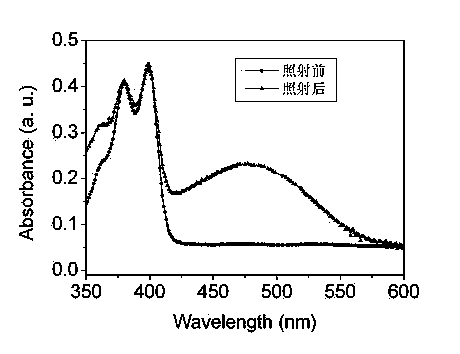
[0042] After the product is pressed into a film by a flat vulcanizer, the film ...
Embodiment 2
[0044] In a 100ml three-necked flask equipped with a reflux condenser and a dropping funnel, add 1.0g of hydrogen-containing silicone oil, 18ml of toluene and 0.003g of chloroplatinic acid aqueous solution, and heat it after feeding argon for 15 minutes. When the temperature rises to 80 o At C, add 0.2g monomer dropwise 2 and 0.08g monomer 4 10ml of the toluene mixed solution was dropped within 1.5 hours, and then continued to be heated to reflux, and the reaction was stopped after 3 hours. Distill under reduced pressure to remove the remaining toluene, and dissolve the residue in a small amount of tetrahydrofuran solvent, remove the catalyst through an aluminum oxide column, and settle in cold methanol for 3 times to obtain a photochromic elastomer. The product is a solid substance with very low hardness , 0.98g, yield 76.5%. The structural formula of the product is as follows:
[0045]
Embodiment 3
[0047] In a 100ml three-necked flask equipped with a reflux condenser and a dropping funnel, add 1.2g of hydrogen-containing silicone oil, 25ml of toluene and an aqueous solution of 0.003g of chloroplatinic acid, and heat it after feeding argon for 15 minutes. 45 o At C, add 0.25g monomer dropwise 3 and 0.1g monomer 4 The toluene mixed solution of 15ml is dripped within 2.5 hours, then continues to be heated to reflux, stops reaction after 3 hours. Distill under reduced pressure to remove the remaining toluene, and dissolve the residue in a small amount of tetrahydrofuran solvent, remove the catalyst through an aluminum oxide column, and settle in cold methanol for 3 times to obtain a photochromic elastomer. The product is a solid substance with very low hardness , 1.28g, yield 82.6%. The structural formula of the product is as follows:
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com