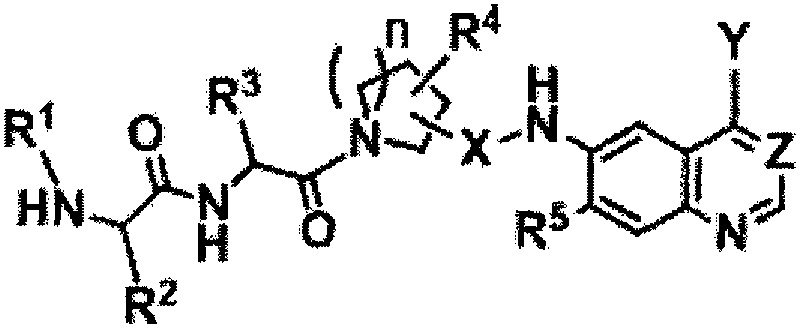
Quinoline or quinazoline derivatives with apoptosis inducing activity on cells
A technology of quinazoline and derivatives, applied in the field of novel quinoline or quinazoline derivatives, can solve the problems of low cell permeability, insurmountable and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 2
[0223] Preparation Example 2: Preparation of (S)-2-((S)-2-(tert-butoxycarbonyl(methyl)amino)propionamido)-3,3-dimethylbutanoic acid
[0224] Preparation of (S)-benzyl 2-(tert-butoxycarbonylamino)-3,3-dimethylbutanoic acid
[0225] Boc-Tle-OH (4.8 g, 22.3 mmol) was dissolved in dichloromethane (50 mL), and EDCI (4.3 g, 44.6 mmol), DMAP (0.6 g, 4.46 mmol), DIPEA (16 mL, 89.2 mmol) and benzyl alcohol (5 mL, 44.6 mmol). The mixture was stirred at room temperature for 12 hours. The mixture was washed several times with 5% aqueous citric acid. The organic layer was dried over sodium sulfate, filtered and distilled under reduced pressure to give the title compound (6.7 g, 99%) as a yellow oil.
[0226] MS (ESI + , m / z): 322[M+H] +
[0227] Preparation of (S)-benzyl 2-amino-3,3-dimethylbutyrate hydrochloride
[0228] The compound (6.7 g, 22.3 mmol) obtained in was dissolved in 4M HCl / dioxane (17 mL) solution, and the mixture was stirred at room temperature for 1 hr. The...
Embodiment 1
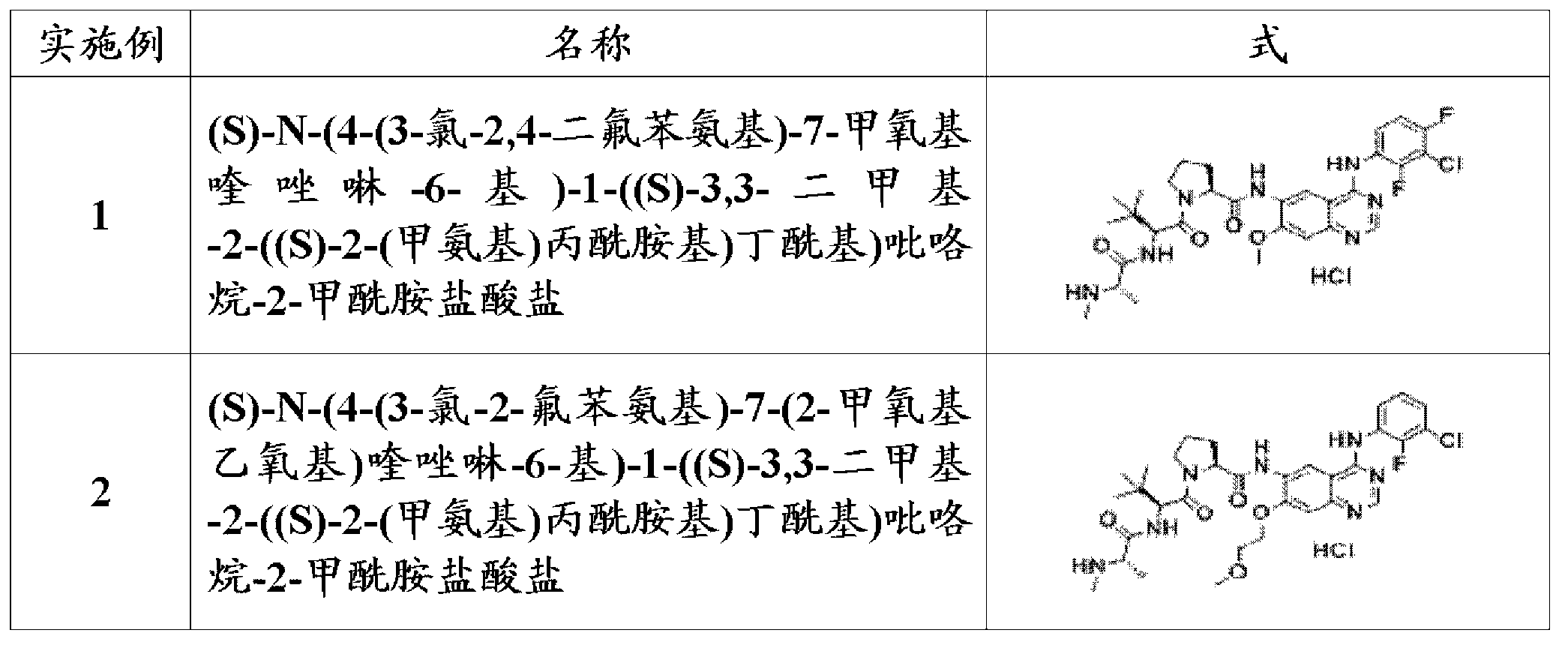
[0236] Example 1: Preparation of (S)-N-(4-(3-chloro-2,4-difluoroanilino)-7-methoxyquinazolin-6-yl)-1-((S)- 3,3-Dimethyl-2-((S)-2-(methylamino)propionamido)butyryl)pyrrolidine-2-carboxamide hydrochloride
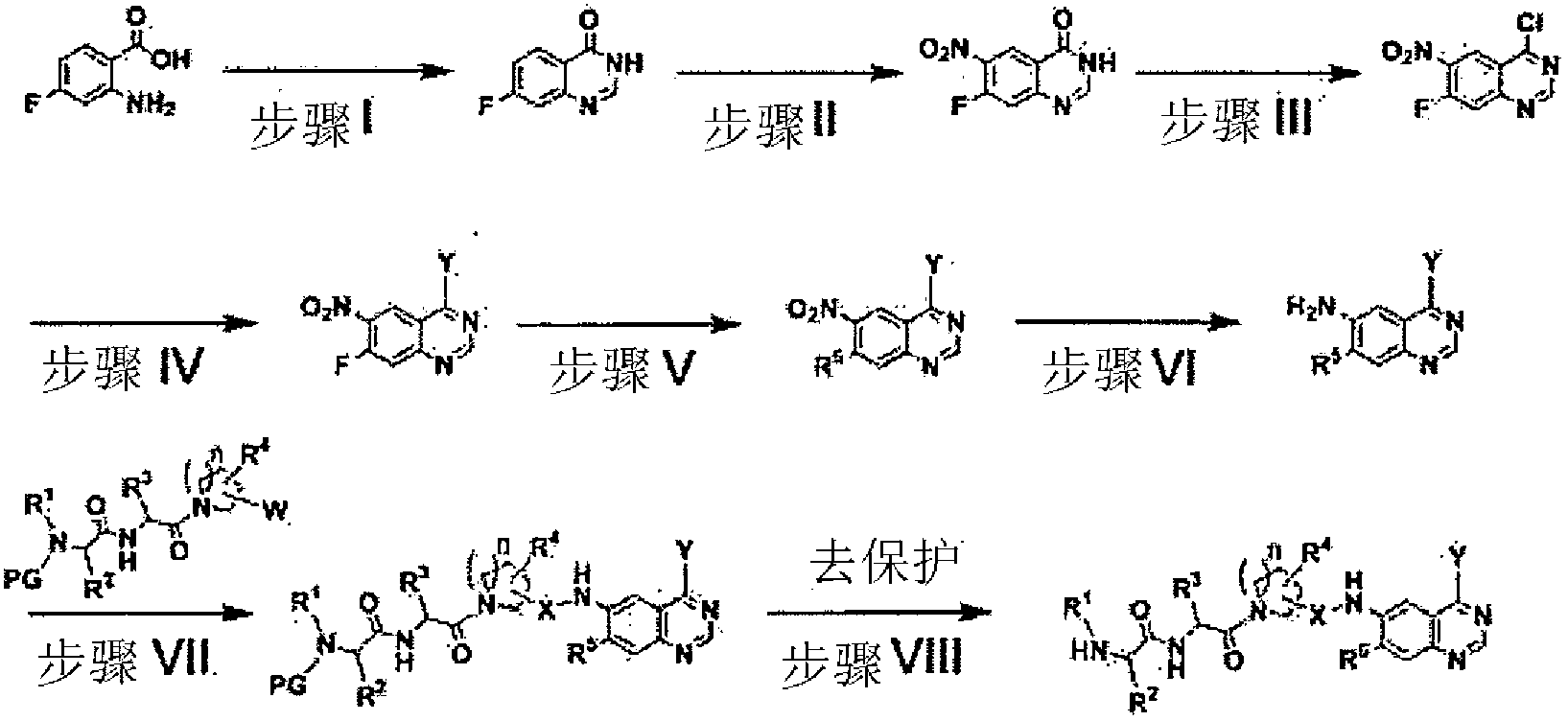
[0237] Preparation of 7-fluoro-3H-quinazolin-4-one
[0238] 2-Amino-4-fluorobenzoic acid (100 g, 0.64 mol) and formamide (154 mL, 3.87 mol) were mixed with a catalytic amount (1 mL) of N,N-dimethylformamide. The mixture was heated to 180°C and stirred for a further 14 hours. The mixture was cooled to room temperature, and distilled water (1000 mL) was added thereto. The mixture was stirred for 30 minutes and filtered to give the title compound (86 g, 81.3%).
[0239] 1 H NMR (300MHz, CDCl 3 ): δ12.34(s, 1H), 8.19-8.12(m, 2H), 7.46-7.34(m, 2H)
[0240] MS (ESI + , m / z): 165[M+H] +
[0241] Preparation of 7-fluoro-6-nitro-3H-quinazolin-4-one
[0242] The compound (25 g, 152 mmol) obtained in was added dropwise to a solution of sulfuric acid (50 mL) and nitric a...
Embodiment 2
[0269] Example 2: Preparation of (S)-N-(4-(3-chloro-2-fluoroanilino)-7-(2-methoxyethoxy)quinazolin-6-yl)-1-( (S)-3,3-Dimethyl-2-((S)-2-(methylamino)propionylamido)butanoyl)pyrrolidine-2-carboxamide hydrochloride
[0270] Repeat except that 2-fluoro-4-chloro-aniline was used instead of 3-chloro-2,4-difluoro-aniline in and 2-methoxyethanol was used instead of NaOMe in The method of Example 1 was used to obtain the title compound (300mg, 9%).
[0271] 1 H NMR (300MHz, DMSO-d6): δ9.76(s, 1H), 9.32(m, 1H), 8.89(s, 1H), 8.78(m, 2H), 8.60(d, 1H), 7.63(t , 1H), 7.52(m, 2H), 7.35(m, 1H), 4.80(m, 1H), 4.43(m, 1H), 4.41(m, 2H), 3.99(m, 2H), 3.88(s, 3H), 3.80(m, 4H), 1.99(m, 6H), 1.35(d, 3H), 1.01(s, 9H)
[0272] MS (ESI + , m / z): 658[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com