Version control for medical anesthesia devices
A medical device, version of technology, applied in the fields of medical technology and electronics or informatics, that can solve problems such as error-prone, time-consuming, delay time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
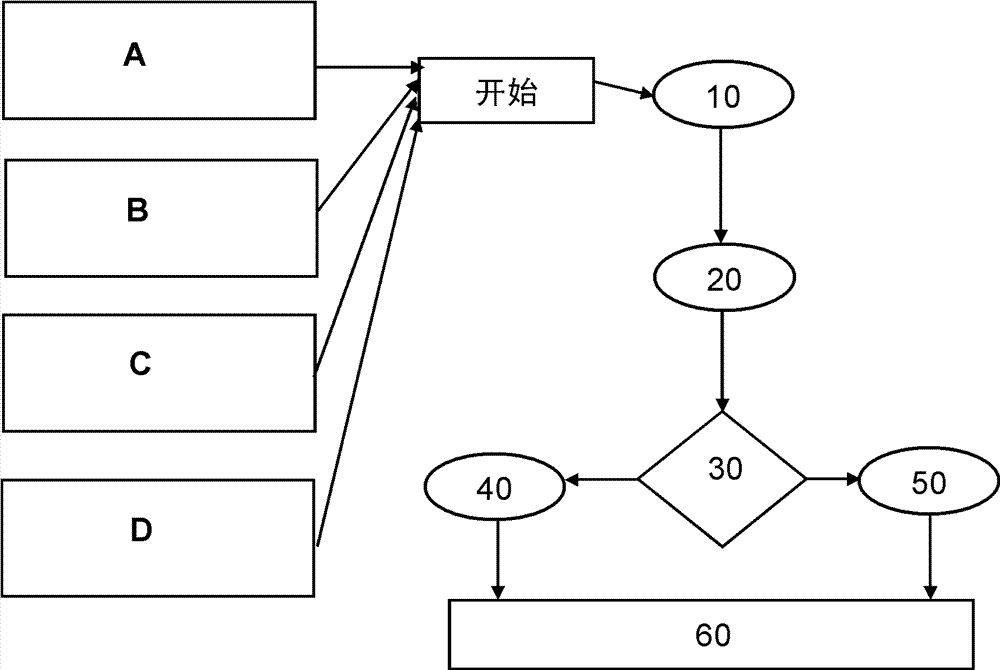
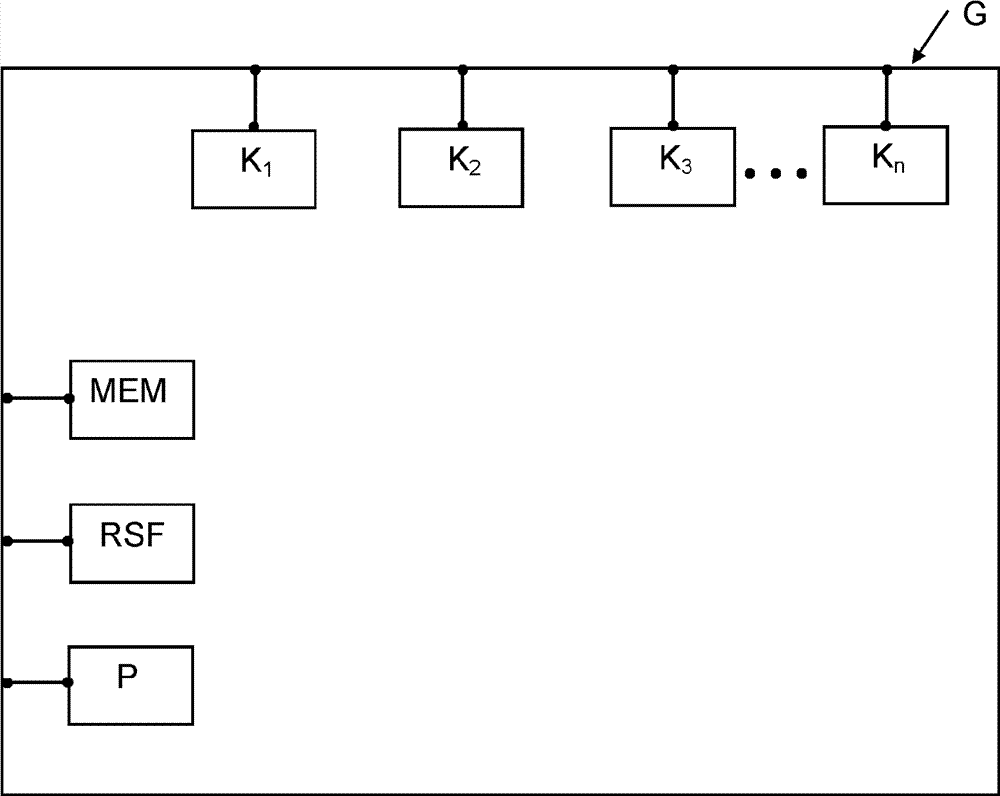
[0058] figure 1 The structure of a medical technology device expanded according to the invention, in particular an anesthesia device, is shown in schematic form, which is designated by the reference symbol G in the figure. The anesthesia device G comprises a plurality of components and / or electronic assemblies K, which are controlled and / or operated via a computer program, for example for measuring flow and pressure, for measuring gas concentrations of different gases (partially designed in multiples), with Such components and / or electronic components for RFID reading and analysis, for measuring physiological data of a patient, and / or power supply parts. The teaching according to the invention can also be applied, for example, to the following components K: for a flow meter (rotameter) for determining the gas flow, for an ORC system (Oxygen Ratio Controller) or, for example, a sensitive The improved design of the ORC also applies (depending on the orientation of the device G)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com