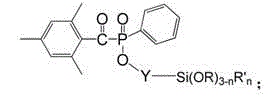
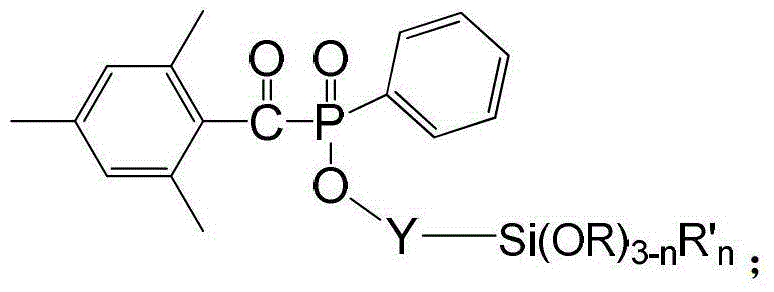
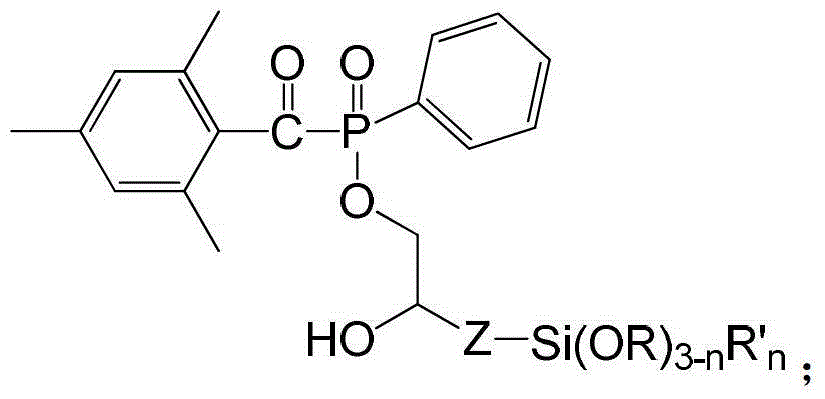
Siloxane-group-containing long-wave-absorbing photoinitiator and preparation method thereof
A siloxane group, light-absorbing technology, used in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, coatings, etc. , Improve the interface photo-initiated efficiency, improve the effect of adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Take 28.8g (0.1mol) of 2,4,6-trimethylbenzoylphenylphosphonic acid in a three-necked flask, dissolve it in 100ml of toluene, add γ-(2,3-epoxypropoxy)propyltrimethyl Oxysilane (KH-560) 23.6g (0.1mol), stirred and heated at 55°C for 2 hours, detected by infrared spectroscopy, the reaction raw material was at 913cm -1 The characteristic absorption peak of epoxy disappears. Rotary evaporation under reduced pressure, remove solvent, obtain product 52.3g, product proton nuclear magnetic spectrum (CDCl 3 , TMS) δ (ppm): 0.54(t,2H), 1.52(m,2H), 2.13(s,3H), 2.33(s,6H), 3.37(t,2H), 3.51(s,9H), 3.44-3.72 (m, 1H+2H), 4.19 (d-d, 2H), 6.79 (s, 2H), 7.34 (m, 2H), 7.65 (m, 1H), 7.71 (m, 2H).
Embodiment 2
[0042] Take 28.8g (0.1mol) of 2,4,6-trimethylbenzoylphenylphosphonic acid in a three-necked flask, dissolve it in 100ml of toluene, add 3-[(2,3)-glycidoxy]propane 22.0g (0.1mol) of methyldimethoxysilane, stirred and heated at 55°C for 2 hours, detected by infrared spectroscopy, the reaction raw materials were at 913cm -1 The characteristic absorption peak of epoxy disappears. Rotary evaporation under reduced pressure, remove solvent, obtain product 50.6g, product proton nuclear magnetic spectrum (CDCl 3 , TMS) δ (ppm): 0.18(s,3H), 1.01(t,2H), 1.54(m,2H), 2.13(s,3H), 2.33(s,6H), 3.36(t,2H), 3.53(s,6H),3.44-3.72(m,1H+2H),4.18(d-d,2H),6.79(s,2H),7.34(m,2H),7.65(m,1H),7.71(m,2H ).
Embodiment 3
[0044] Take 28.8g (0.1mol) of 2,4,6-trimethylbenzoylphenylphosphonic acid in a three-necked flask, dissolve it in 100ml of toluene, add 22.0g of 5,6-epoxyhexyltrimethoxysilane ( 0.1mol), stirred and heated at 55°C for 2 hours, detected by infrared spectroscopy, the reaction raw materials were at 908cm -1 The characteristic absorption peak of epoxy disappears. Rotary evaporation under reduced pressure, remove solvent, obtain product 50.7g, product proton nuclear magnetic spectrum (CDCl 3 , TMS) δ (ppm): 0.59(t, 2H), 1.29-1.38(m, 2H+2H), 1.46(d-d, 2H), 2.13(s, 3H), 2.33(s, 6H), 3.34(m ,1H), 3.53(s,9H), 4.18(d-d,2H), 6.79(s,2H), 7.34(m,2H), 7.65(m,1H), 7.71(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com