Method for continuous production of epoxy chloropropane through reaction-separation coupling
A technology of epichlorohydrin and chloropropene, applied in the direction of organic chemistry, etc., can solve the problems of affecting the reaction conversion rate, difficult and unfavorable product separation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The qualitative and quantitative detection method of the reaction substrate and product in this example is: using SE-54 (30 m×0.25 mm×0.5 μm) quartz capillary column, the temperature of the vaporization chamber is 200 °C; the temperature of the column is 130 °C; the temperature of the FID detector is 260 °C ; Carrier gas: high-purity N 2 ; Column flow rate: 0.66 mL / min; Split ratio: 60:1.
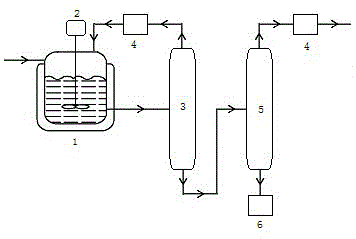
[0015] Pump 38.26 g (0.5 mol) of allyl chloride and 69.06 g (0.5 mol) of peroxybenzoic acid into reaction kettle 1, heat to a reaction temperature of 80 °C, and stir vigorously for 2.5 h. In the distillation tower 3, the light components of allyl chloride and peroxybenzoic acid at the top of the rectification tower 3 are refluxed in the reactor 1 after being condensed, and the heavy components at the bottom of the tower are pumped into the secondary rectification tower 5 for secondary rectification , the light component epichlorohydrin at the top of the tower was condensed by the to...
Embodiment 2
[0017] Reaction substrate and product qualitative and quantitative detection method and operation are all the same as in Example 1, and the implementation steps of changing the reactant molar ratio and each operating parameter are as follows:
[0018] Pump 38.26 g (0.5 mol) of allyl chloride and 207.18 g (1.5 mol) of peroxybenzoic acid into reaction kettle 1, heat to the reaction temperature of 60 °C, and stir vigorously for 1 h. In the distillation tower 3, the light components of allyl chloride and peroxybenzoic acid at the top of the rectification tower 3 are refluxed in the reactor 1 after being condensed, and the heavy components at the bottom of the tower are pumped into the secondary rectification tower 5 for secondary rectification , the light component epichlorohydrin at the top of the tower was condensed by the top condenser 4 to collect 42.42 g of high-purity product epichlorohydrin, with a yield of 91.7%. .
Embodiment 3
[0020] Reaction substrate and product qualitative and quantitative detection method and operation are all the same as in Example 1, and the implementation steps of changing the reactant molar ratio and each operating parameter are as follows:
[0021] Pump 38.26 g (0.5 mol) of allyl chloride and 345.30 g (2.5 mol) of peroxybenzoic acid into Reactor 1, heat to a reaction temperature of 45 °C, and stir vigorously for 4 h. In the distillation tower 3, the light components of allyl chloride and peroxybenzoic acid at the top of the rectification tower 3 are refluxed in the reactor 1 after being condensed, and the heavy components at the bottom of the tower are pumped into the secondary rectification tower 5 for secondary rectification , the light component epichlorohydrin at the top of the tower was condensed by the top condenser 4 to collect 44.78 g of high-purity product epichlorohydrin, with a yield of 96.8%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap