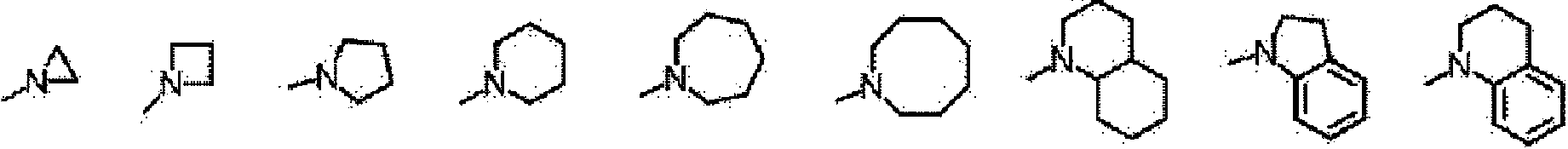Colorant dispersion liquid
A technology of colorant and dispersion liquid, which is applied in the fields of optics, instruments, optical mechanical equipment, etc., can solve the problem that the coloring curable composition may not necessarily have storage stability, and achieve the effect of excellent storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0538] The following examples are enumerated and the present invention is further specifically described, but the present invention is not limited to the following examples, and it can also be implemented by suitably changing within the scope of the aforementioned and later-mentioned gist, and these are all included in the technical scope of the present invention Inside. Unless otherwise stated, "%" and "part" in an example are mass % and a mass part. In the following synthesis examples, the compounds were identified by mass spectrometry (LC: 1200 model manufactured by Agilent; MASS: LC / MSD model manufactured by Agilent).
Synthetic example 1
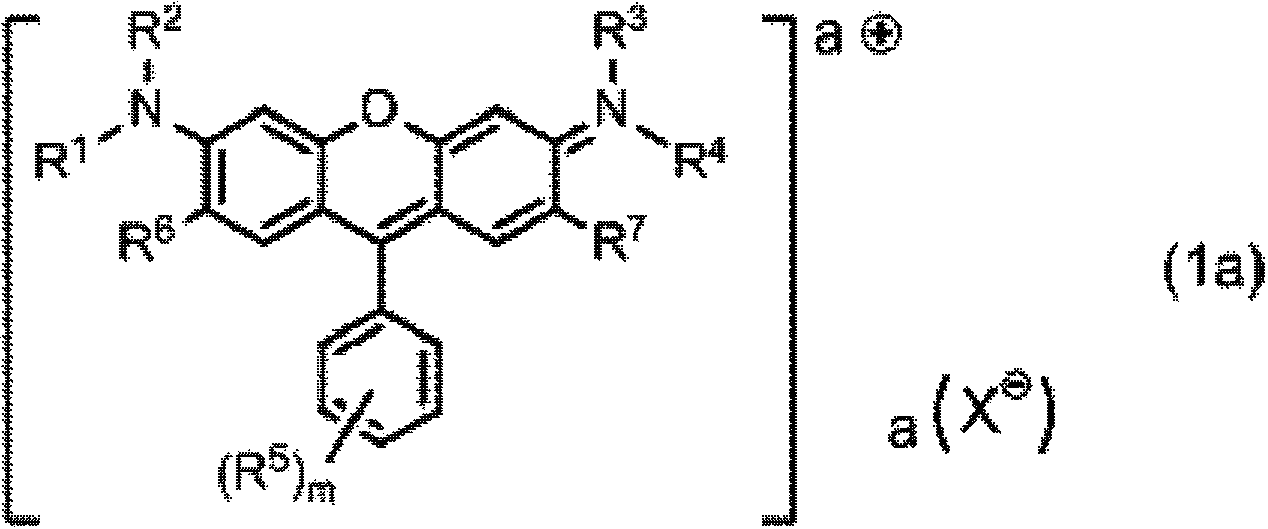
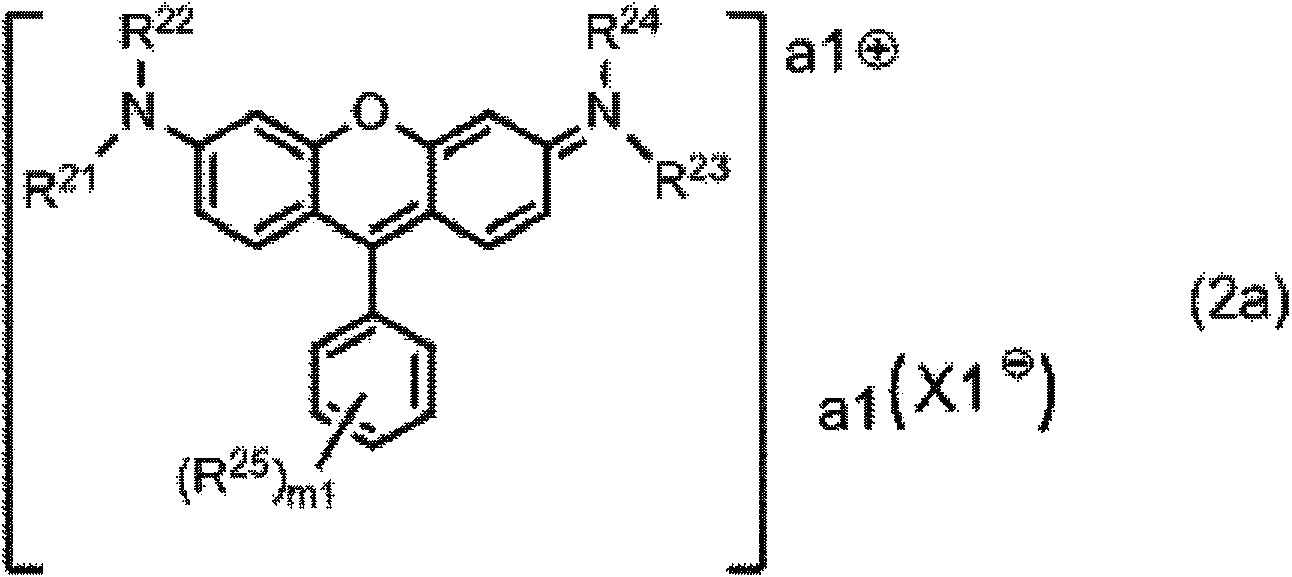
[0540] 20 parts of the compound represented by the formula (1x) and 200 parts of N-ethyl-o-toluidine (manufactured by Wako Pure Chemical Industries, Ltd.) were mixed under light-shielding conditions, and the resulting solution was stirred at 110° C. for 6 hours. After cooling the obtained reaction liquid at room temperature, it was added to a liquid mixture of 800 parts of water and 50 parts of 35% hydrochloric acid, and stirred at room temperature for 1 hour to precipitate crystals. The precipitated crystal was taken out as the residue of suction filtration and dried to obtain 24 parts of the compound represented by the formula (1-24). The yield is 80%.
[0541]
[0542] Identification of compounds represented by formula (1-24)
[0543] (mass analysis) ionization mode = ESI + : m / z=[M+H] + 603.4
[0544] Exact Mass: 602.2
[0545] [Synthesis Example 2]
[0546] A compound represented by the formula (1-32) was obtained in the same manner as in Synthesis Example 1 exce...
Synthetic example 10
[0588] Mix 1.29 parts of bis(3-amino-4-hydroxyphenyl)sulfone (manufactured by Tokyo Chemical Industry Co., Ltd.), 4-(bis(2-ethylhexyl)amino)salicylaldehyde (based on JP2007-508275-A The disclosed method is synthesized) 3.31 parts, 0.385 parts of benzoic acid (Tokyo Chemical Industry (Co., Ltd.), 1-pentanol (Tokyo Chemical Industry (Co., Ltd.) )) 1.06 parts, stirred at 120°C for 3 hours. 3.29 parts of 4-(bis(2-ethylhexyl)amino) salicylaldehyde (synthesized based on the method disclosed in JP2007-508275-A) and benzoic acid (manufactured by Tokyo Chemical Industry Co., Ltd.) were added to the reaction solution. 0.406 parts, 1-pentanol (manufactured by Tokyo Chemical Industry Co., Ltd.) 11.9 parts, and ethyl cyanoacetate (manufactured by Tokyo Chemical Industry Co., Ltd.) 1.04 parts were stirred at 120° C. for 12 hours. After cooling the above reaction liquid to room temperature, it added to 450 parts of methanol, and removed the supernatant to obtain a precipitate. 5.00 parts o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| amine value | aaaaa | aaaaa |
| amine value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com