Modifier of 5-HT2A serotonin receptor
A halogen, solvate technology, applied in the field of regulators of 5-HT2A serotonin receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
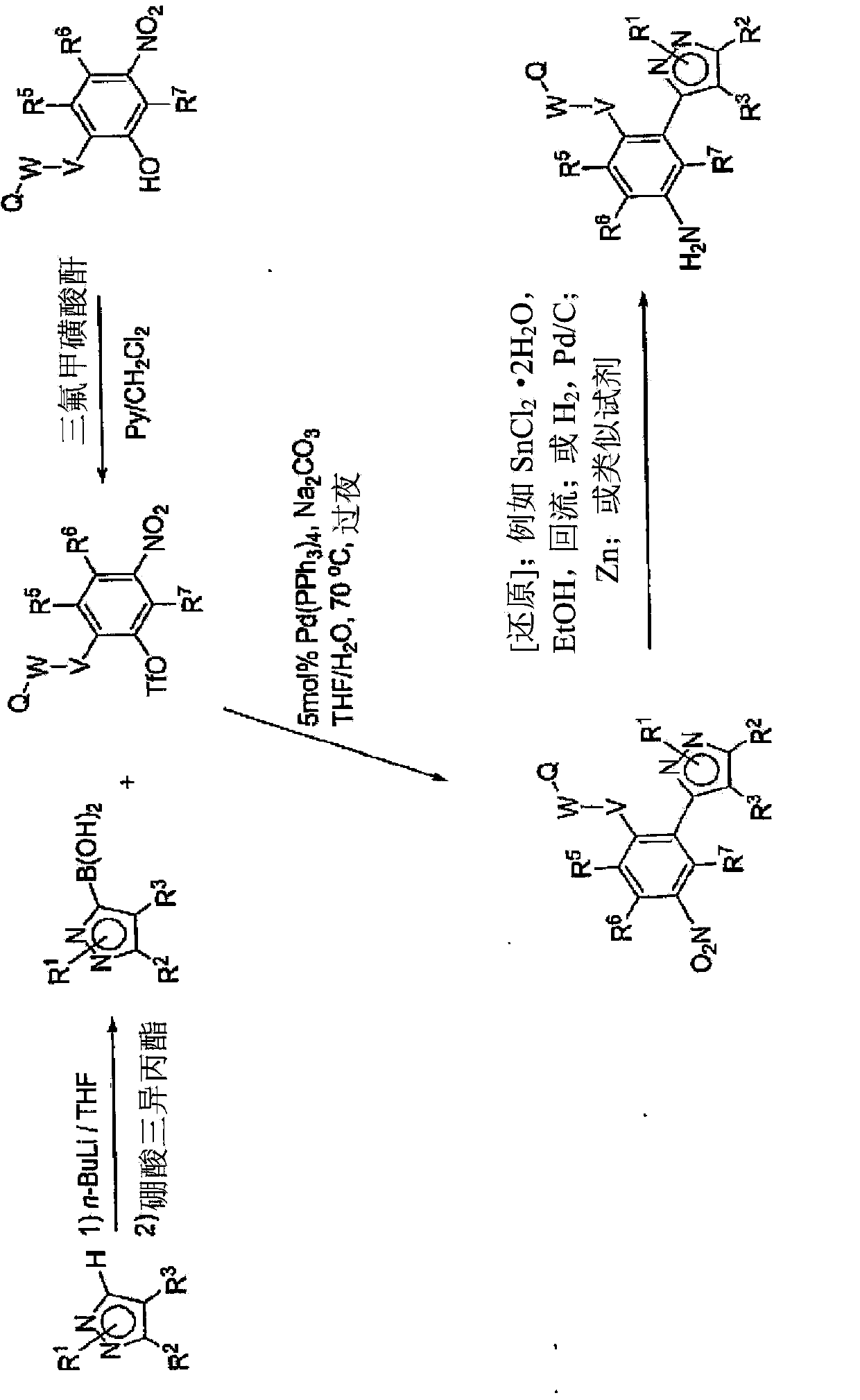
[0489] for the active content of 125 The synthetic method that I incorporates target molecule comprises:
[0490] A. Sandmeyer and similar reactions - this procedure converts an aryl or heteroaryl amine to a diazonium salt such as tetrafluoroborate and then uses Na 125 I converted to 125 I labeled compound. A representative procedure is reported by Zhu, D.-G. and co-workers in J. Org. Chem. 2002, 67, 943-948.
[0491] B. Phenol ortho 125 Iodination - This procedure allows 125 I was incorporated in the ortho position to the phenol as reported by Collier, T.L. and coworkers in J. labeled Compd Radiopharm. 1999, 42, S264-S266.
[0492] C. Aryl bromides and heteroaryl bromides with 125 I Interchange - This method is usually a two-step procedure. The first step is to use such as Pd to catalyze the reaction [ie Pd(Ph 3 P) 4 ] or via aryllithium or heteroaryllithium in a trialkyltin halide or hexaalkylditin [e.g., (CH 3 ) 3 SnSn(CH 3 ) 3 ] to convert aryl and heteroaryl ...
example 1
[0498] Example 1: Synthesis of Compounds of the Invention.
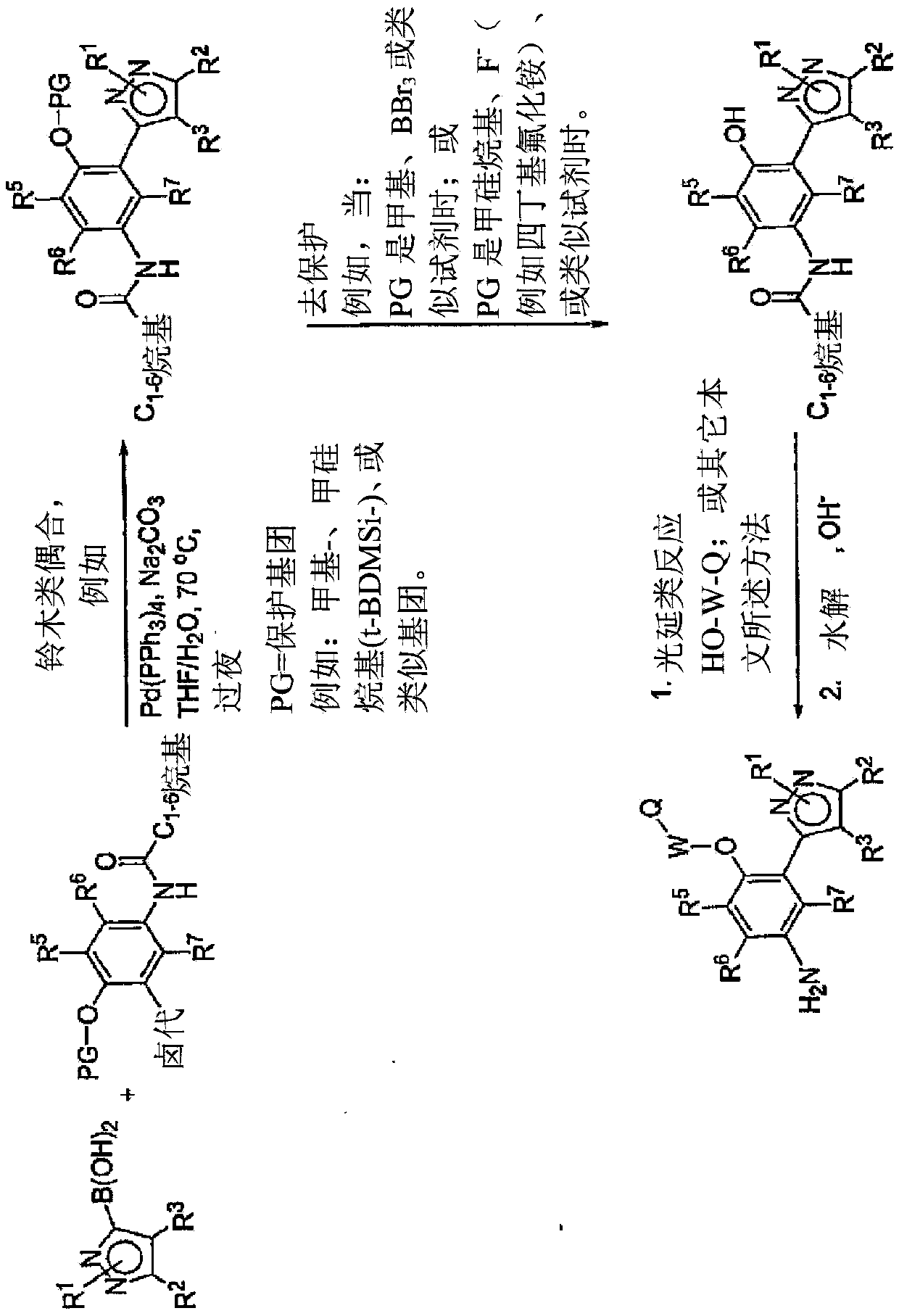
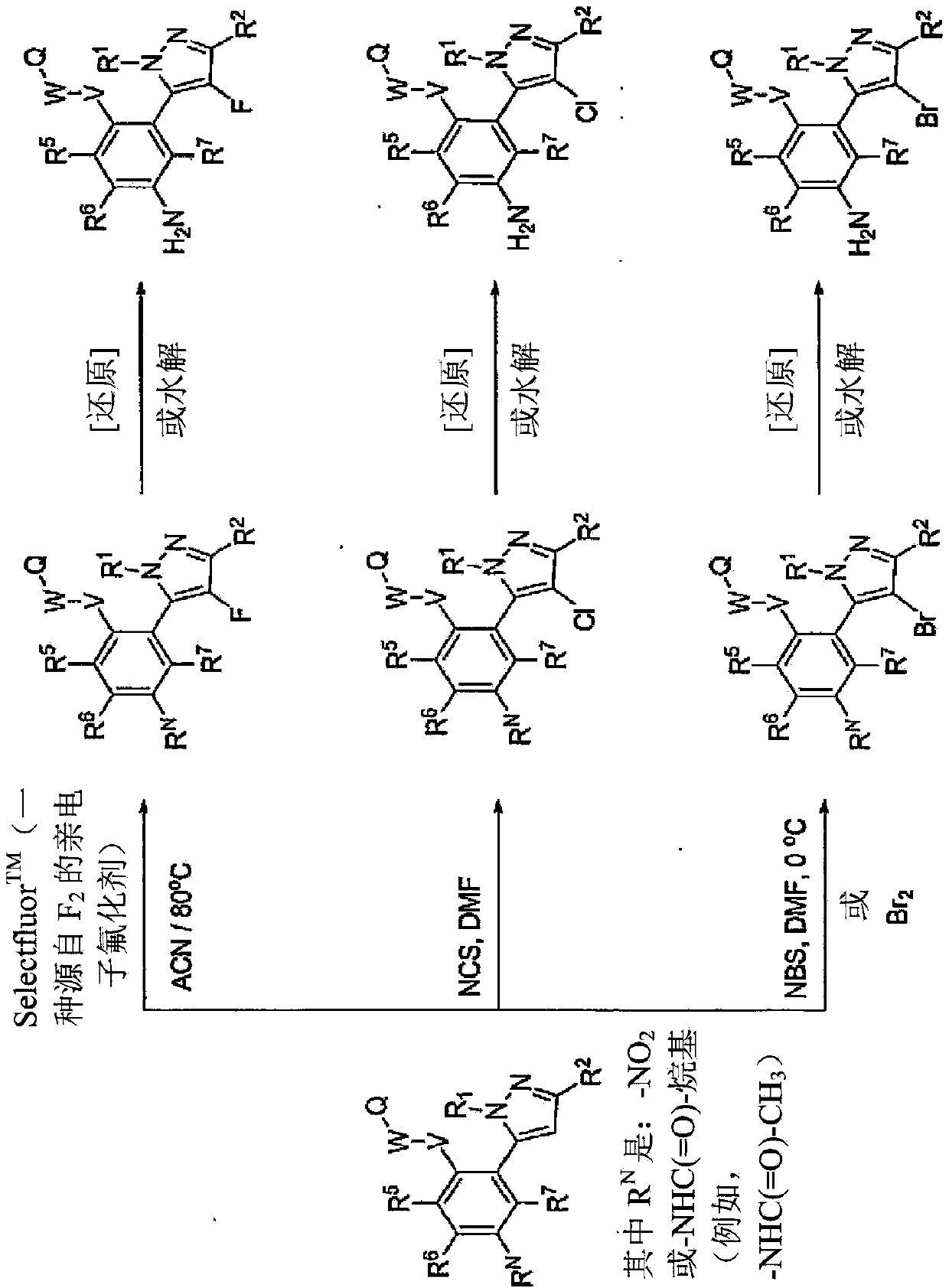
[0499] Exemplary syntheses of compounds of the invention are shown in Figures 1 to 8 , where the symbols used have the same definitions throughout this disclosure.
[0500] The compounds of the invention and their synthesis are further illustrated by the following examples. The following examples are provided to further define the invention without, however, limiting the invention to the details of these examples. Compounds described herein above and below are named according to CS Chem Draw Ultra version 7.0.1 or AutoNom2000. Common names are used in certain instances and it is understood that those skilled in the art will recognize these common names.
[0501] Chemical Properties: Proton NMR ( 1 H NMR) spectra were obtained on a Varian Mercury Vx-400 equipped with 4-nuclear autotransfer probe and z-gradient or Bruker Avance equipped with QNP (Quad nuclear probe) or BBI (broadband inverse probe) and z-gradient ...
example 11
[0504] Example 1.1: Preparation of N-{3-(4-chloro-2-methyl-2H-pyrazol-3-yl)-4-[2-(1-methyl-piperidin-4-ylamino)-ethyl Oxy]-phenyl}-3-trifluoromethyl-benzamide (compound 45).
[0505] To N-(4-(2-bromo-ethoxy)-3-(4-chloro-2-methyl-2H-pyrazol-3-yl)phenyl)-3-trifluoromethyl-benzyl To a solution of amide (0.050 g, 99.5 μmol) in DMA (3 mL) was added 1-methyl-piperidin-4-ylamine (17.0 mg, 149 μmol) and N,N-diisopropylethylamine (34.7 μL, 199 μmol). The reaction mixture was heated at 150° C. for 30 minutes in a thick-walled sealed tube under microwave irradiation and then purified by preparative HPLC. The corresponding fractions were collected and lyophilized to afford the diTFA salt of the title compound (hygroscopic) as a white solid in 40.1% yield. LCMS m / z(%)=536(M+H, 35 Cl,100),538(M+H, 37 Cl, 43). 1 H NMR (400MHz, DMSO-d 6 )δ10.56(s,1H),9.11-8.92(m,2H),8.31-8.24(m,2H),8.01-7.95(m,2H),7.80(t,J=7.8Hz,1H),7.73 (d,J=2.6Hz,1H),7.71(s,1H),7.31(d,J=9.1Hz,1H),4.37-4.23(m,2H),3.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com