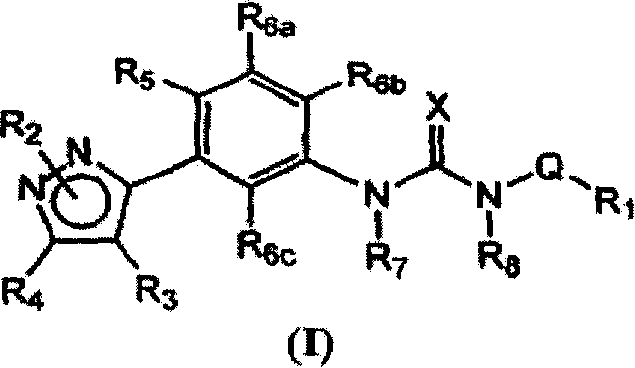
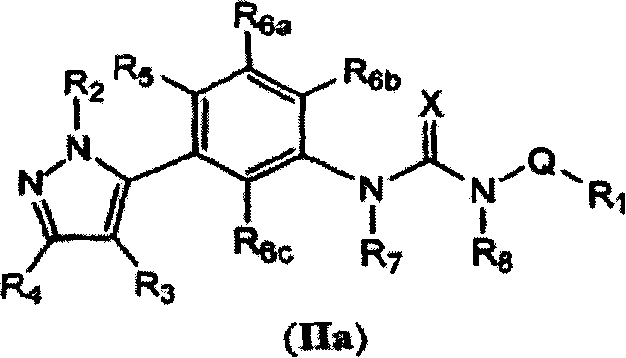
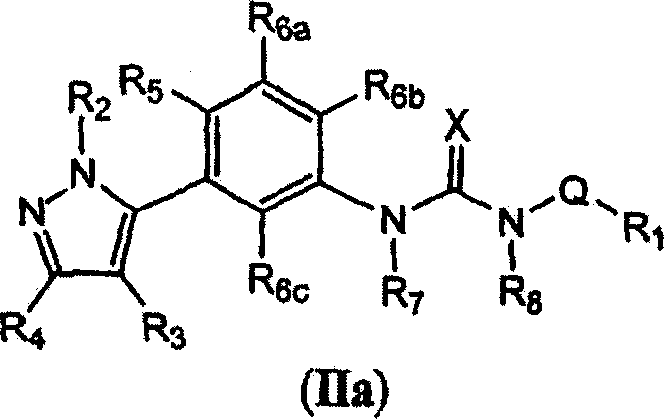
Diaryl and arylheteroaryl urea derivatives as modulators of the 5-HT2A serotonin receptor useful for the prophylaxis and treatment of disorders related therto
A technology of solvates and alkyls, applied in anti-inflammatory agents, metabolic diseases, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0618] for the active content of 125 Synthetic methods for I to be incorporated into target molecules include:
[0619] A. The Sandmeyer reaction and similar reactions - This procedure converts an aryl or heteroaryl amine to a diazonium salt, such as tetrafluoroborate, and subsequently uses Na 125 I convert it into 125 I labeled compound. Representative procedures are reported by Zhu, D.-G. and co-workers in J. Org. Chem., 2002, 67, 943-948.
[0620] B. Ortho-iodine of phenols [ 125 I] Reaction - This procedure allows the 125 I was incorporated in the ortho position of the phenol as reported by Collier, T.L. and co-workers, J. Labeled Compd. Radiopharm., 1999, 42, S264-S266.
[0621] C. use 125 I Substitution of Brominated Aryl and Heteroaryl - This method generally consists of a two-step process. The first step is: in trialkyltin halide or hexaalkylditin [eg (CH 3 ) 3 SnSn(CH 3 ) 3 ] in the presence of, for example, Pd-catalyzed reactions [ie, Pd(Pd(Ph 3 P) 4 )] ...
example 1
[0627] Example 1 Synthesis of Compounds of the Invention.
[0628] The compound syntheses illustrated in the present invention are shown in Figures 17 to 21 and Figures 29 to 34, where the symbols have the same definitions as used throughout this disclosure.
[0629] The compounds of the present invention and their synthesis are further illustrated by the following examples. However, the following examples are provided to further illustrate the invention without limiting the invention to these particular examples. Compounds described herein (above and below) were named according to CS Chem DrawUltra Version 7.0.1, AutoNom version 2.2. In certain instances, common names are used and it is understood that such common names should be recognized by those skilled in the art.
[0630] Chemistry: on a Varian Mercury Vx-400 equipped with 4-nuclei automatic switchable probe and z-gradient or on a Varian equipped with QNP (Quad Nucleus Probe) or BBI (Broad Band Inverse) and z-gradient...
example 11
[0633] Example 1.1: Preparation of intermediate 3-(4-bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxy-aniline.
[0634] To a stirred solution of 4-bromo-5-(2-methoxy-5-nitro-phenyl)-1-methyl-1H-pyrazole (1.799 g, 5.76 mmol) in EtOH (20 mL) was added SnCl 2 2H 2 O (5.306 g, 23.05 mmol, 4.0 equiv), the mixture was stirred at reflux for 2 hours, and the EtOH was removed under vacuum. The resulting solid was dissolved in EtOAc, 1N NaOH (30 mL) was added, and the mixture was stirred overnight. The white precipitate was filtered off through celite, and the aqueous phase was extracted with EtOAc (3 x 80 mL). with anhydrous MgSO 4 The combined organic phases were dried, filtered and evaporated. through SiO 2 The crude reaction mixture was purified by column chromatography (eluent: EtOAc / hexane = 1 / 3, then 1 / 1) to yield 3-(4-bromo-2-methyl-2H-pyridine as a white solid Azol-3-yl)-4-methoxy-aniline (1.430 g, 5.07 mmol, 88%): LCMS m / z (%) = 282 (M+H 79 Br, 98), 284 (M+H 81 Br, 100). 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com