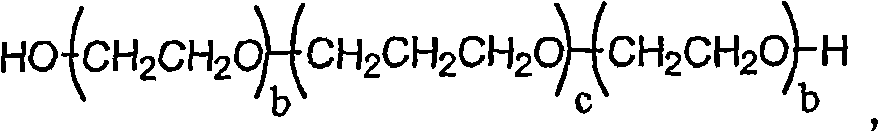Pharmaceutical compositions of a 5-HT2a serotonin receptor modulator useful for the treatment of disorders related thereto
A pharmaceutical composition, composition technology, applied in the direction of drug combination, pharmaceutical formula, medical preparations of non-active ingredients, etc., can solve the problems of weak water solubility, low oral bioavailability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
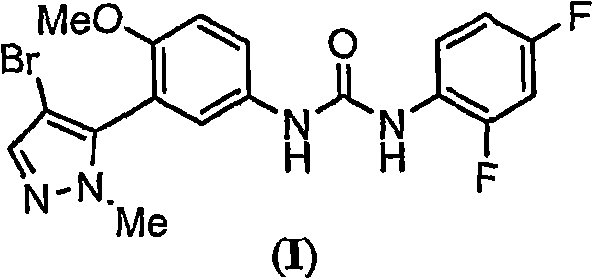
[0338]Example 1: 1-[3-(4-bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxy-phenyl]-3-(2,4-difluoro-phenyl Solubility determination of )-urea in selected excipients
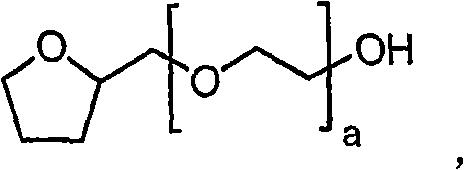
[0339] Add excess 1-[3-(4-bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxy-phenyl]-3-(2,4- Difluoro-phenyl)-urea and excipients to give a suspension. For some excipients, no suspension was observed and therefore solubility was considered greater than a specific volume added, e.g. Transcutol TM P, PEG 300, PEG 600, Tween TM ) 20 and Softigen 767 (see table below). The contents of the bottle were mixed for 30 seconds using a VWR mini vortexter, followed by sonication (Branson 1510) for 1 minute. The bottle was placed in a constant temperature bath (ie, about 25°C) and allowed to equilibrate for no more than 12 hours. The resulting suspensions were transferred to eppendorf tubes each equipped with a 0.2 μm nylon filter (Costar 8168) and centrifuged at 14,000 rpm for 10 minutes. The supernatant from each Eppendorf tube was co...
example 2
[0350] 1-[3-(4-bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxy-phenyl]-3-(2 , Pharmacokinetics of 4-difluoro-phenyl)-urea
[0351] 1-[3-(4-bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxy-phenyl]-3-(2,4- Determination of 1-[3-(4-bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxyl in healthy male and female volunteers after difluoro-phenyl)-urea and Cremephor RH40 Plasma pharmacokinetics of phenyl-phenyl]-3-(2,4-difluoro-phenyl)-urea.
[0352] A single oral dose of 1-[3-(4-bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxy-phenyl]-3-(2 , 4-difluoro-phenyl)-urea
[0353]
example 3
[0355] 1-[3-(4-bromo-2-methyl-2H-pyrazol-3-yl) in male Sprague-Dawley rats after oral administration with various excipients Pharmacokinetics of -4-methoxy-phenyl]-3-(2,4-difluoro-phenyl)-urea
[0356] After oral administration of 10 mg / kg of 1-[3-(4-bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxy-phenyl]-3-(2,4- Determination of 1-[3-(4-bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxyl in male Sprague-Dawley rats following difluoro-phenyl)-urea Plasma pharmacokinetics of phenyl-phenyl]-3-(2,4-difluoro-phenyl)-urea. 1-[3-(4-bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxy-phenyl]-3-(2,4-difluoro-phenyl)- Urea in polyethylene glycol 400 (PEG 400), Labrasol, Cremaphor RH40, 80% Tween 80 and 20% water (Tween), Cremaphor RH40: Labrasol (1:1, v / v), 40% hydroxypropyl- β-cyclodextrin (HPCD) and dimethylacetamide (DMAC) prepared as an aqueous suspension. Dosage formulations are administered via oral gavage tube.
[0357] 1-[3-(4-bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxy-phenyl]-3-(2,4-di Mean pharm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com