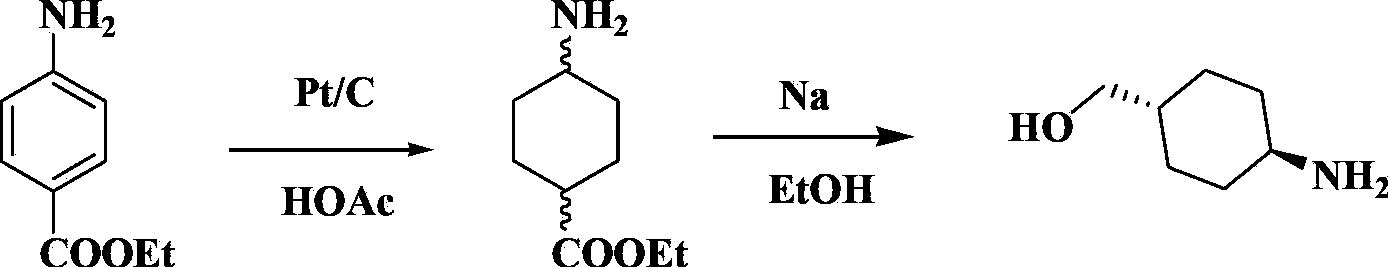
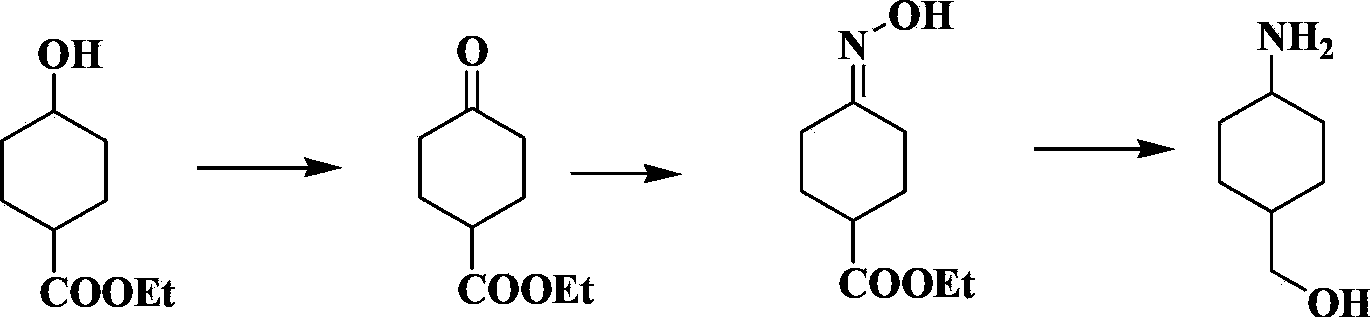
Trans-4-amino cyclohexylmethanol hydrochloride and preparation method thereof
A technology of aminocyclohexyl and hydrochloride, applied in the field of medicine, can solve the problem of large amount of reducing agent, etc., and achieve the effect of simple operation, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
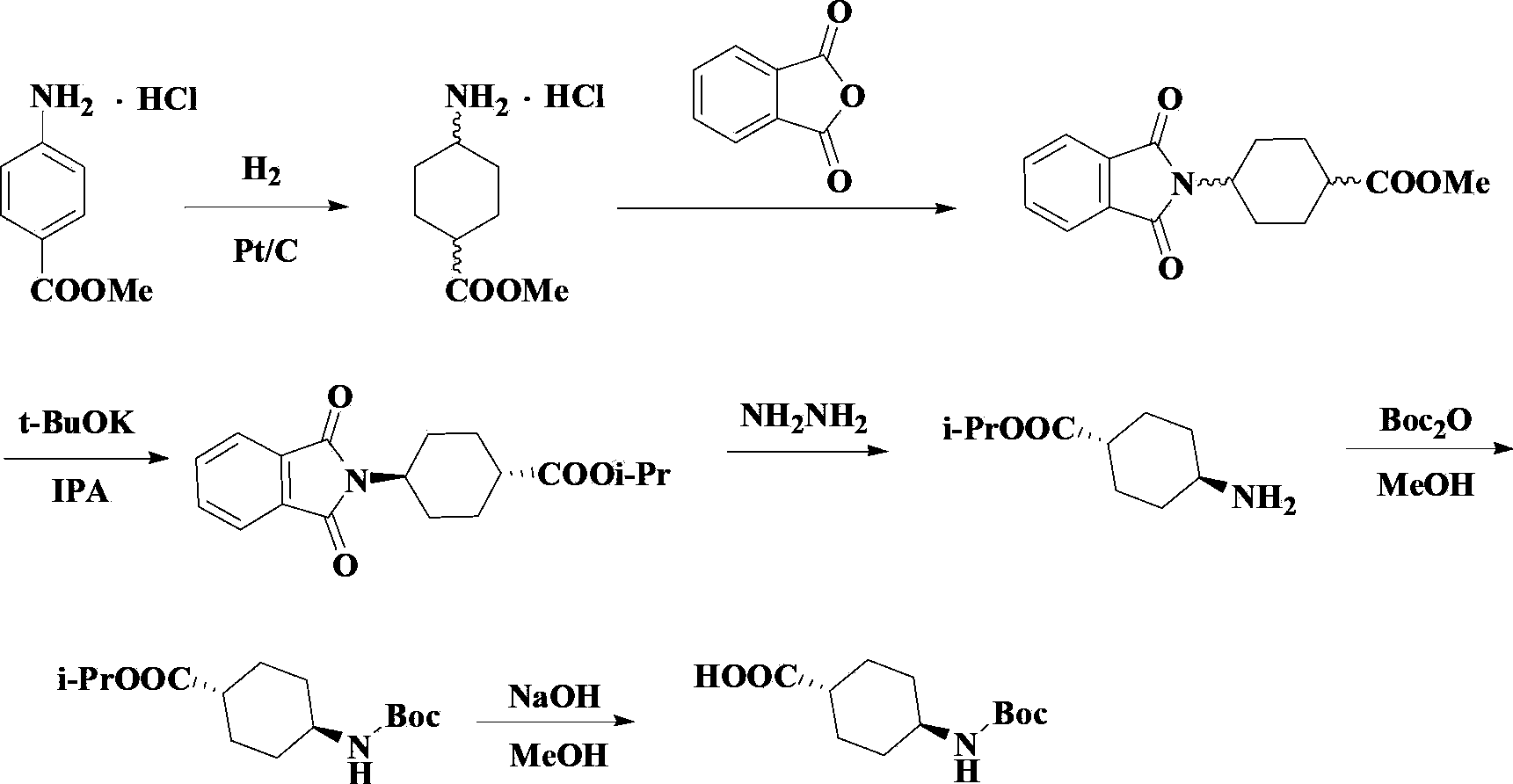
[0053] Example 1: Synthesis of 4-aminocyclohexylcarboxylate (II)
[0054]In a 3L autoclave, add 400.0g of ethyl p-aminobenzoate, 24.1g of 5% ruthenium-carbon, 1600mL of tetrahydrofuran in sequence, nitrogen replacement, temperature 100-150°C, hydrogen filling to make the reaction system pressure 8-15MPa, no pressure drop Reaction 2 ~3h. TLC monitoring, no fluorescence absorption in the central area means the reaction is complete. The catalyst was filtered off to obtain a colorless clear liquid. Tetrahydrofuran was distilled off under reduced pressure and then distilled to obtain 320.0 g of a colorless clear liquid with a yield of 74.3% and a content of ≥98% as determined by GC.
Embodiment 2
[0055] Example 2: Synthesis of 4-substituted phthalimide cyclohexyl carboxylate (III)
[0056] In a 2L three-necked flask, add 85.5g of ethyl 4-aminocyclohexylcarboxylate, 855g of toluene, and 40.4g of triethylamine in sequence, and add 170.5g of tetrachlorophthalic anhydride in batches, and the system becomes a colorless transparent liquid. Heat the water and reflux for 6-10 hours. GC traced to complete conversion of starting material. Cool to room temperature, add 400.0g 3-4% NaOH solution, stir for 10-15min, and separate the toluene layer. Add 400.0 g of 3-4% HCl solution to the toluene layer, stir for 10-15 min, collect the toluene layer, and dry over anhydrous sodium sulfate to obtain a light yellow clear solution. Filter off the desiccant. The solvent was evaporated under reduced pressure to obtain 207.5 g of a colorless or yellow viscous liquid, with a yield of 95.0%, and the content measured by HPLC was ≥99%.
Embodiment 3
[0057] Example 3: Synthesis of trans-4-substituted phthalimide cyclohexyl carboxylate (IV)
[0058] Add 26.6g of potassium tert-butoxide and 600g of tetrahydrofuran into a 2L three-necked flask in sequence, and stir to dissolve. Under the protection of nitrogen, add a mixed solution of 160g compound III and 480g tetrahydrofuran, heat until the solid dissolves, evaporate the solvent tetrahydrofuran, raise the temperature to 180-220°C, and bake at high temperature for 4-20h. Add 2% aqueous solution of glacial acetic acid until the system pH=7, cool down and precipitate white crystals. Inner temperature 0~5℃, heat preservation and stirring for 0.5h, suction filtration to obtain a white flocculent solid, washed with water, dried, and recrystallized in a mixed solvent of cyclohexane and dichloromethane. 140.0 g of white crystals were obtained, the total yield was 83.6%, the content determined by HPLC was ≥99.5%, and the cis-isomer content was lower than the detection limit.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap