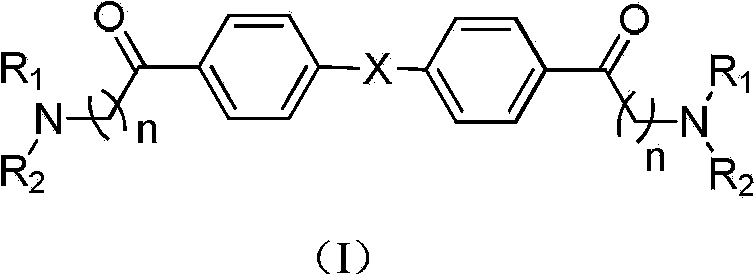
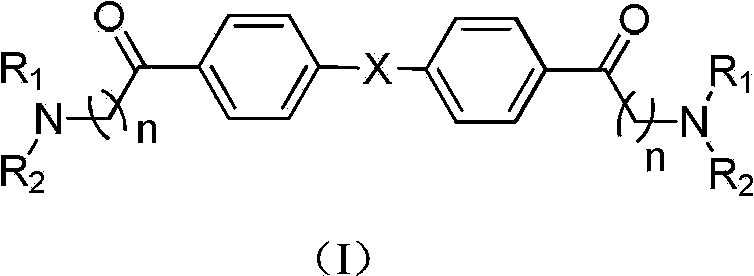
Compound with dual inhibitory activities to acetylcholine esterase and cholinesterase
A compound, selected technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Synthesis of 1,1'-([1,1'-biphenyl]-4,4'-diyl)bis(2-(dimethylamino)ethanone
[0058]
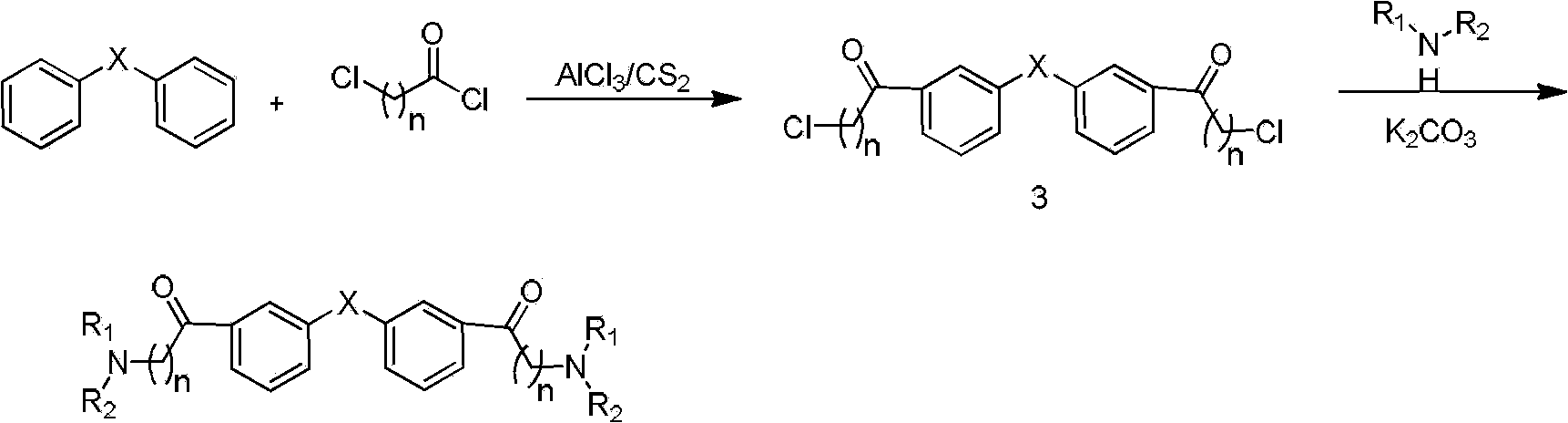
[0059] Add biphenyl (7.71g, 50mmol), anhydrous AlCl 3 (20g, 150mmol), 100ml CS 2 , under mechanical stirring, heated to 50°C, added dropwise chloroacetyl chloride (11.94ml, 150mmol), after addition, raised the temperature to 80°C, reacted for 4h, stopped heating, poured the reaction solution into ice water, stirred, pumped Filter, wash with water, and dry to obtain 10.10 g of a yellow solid (intermediate), with a yield of 66%.
[0060] In a 250 mL dry round bottom flask add K 2 CO 3 (1.38g, 10mmol), KI (0.83g, 5mmol), 20ml of acetonitrile, heated to 50°C, stirred for 30min, then added dimethylamine hydrochloride (0.81g, 10mmol), and the intermediate synthesized above (1.53g , 5mmol). Raise the temperature to 80°C, react for 10h, stop heating, after cooling, concentrate the reaction mixture under reduced pressure, add water to the residue, CH 2 Cl 2 Extracted three times, the ...
Embodiment 2
[0063] Synthesis of 1,1'-([1,1'-biphenyl]-4,4'-diyl)bis(2-(morpholinyl)ethanone
[0064]
[0065] The operating steps were the same as in Example 1, except that dimethylamine hydrochloride was replaced by morpholine: to obtain the title compound as a pale yellow solid. The yield is 65%.
[0066] 1 H-NMR (300MHZ, CDCl 3 )δ: 8.039(d,4H), 7.744(d,4H), 3.781(s,4H), 3.724(t,8H), 2.574(t,8H).
Embodiment 3
[0068] Synthesis of 1,1'-([1,1'-biphenyl]-4,4'-diyl)bis(2-(4-methylpiperazin-1-yl)ethanone
[0069]
[0070] The operation steps were the same as in Example 1, except that dimethylamine hydrochloride was replaced by N-methylpiperazine to obtain the title compound as a pale yellow solid. The yield is 70%.
[0071] 1 H-NMR (300MHZ, CDCl 3 )δ: δ8.106(d,4H),7.706(d,4H),3.859(s,4H),2.654(d,16H),2.323(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com