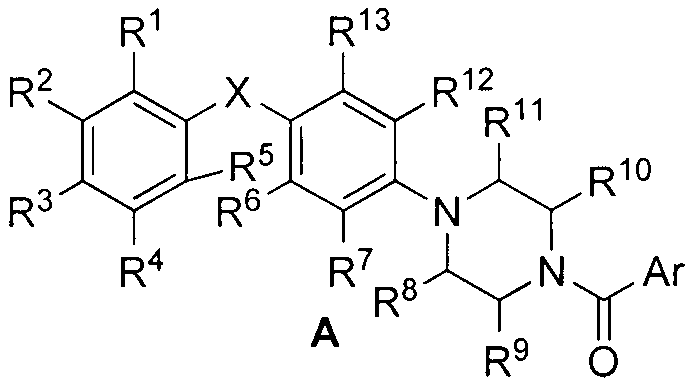
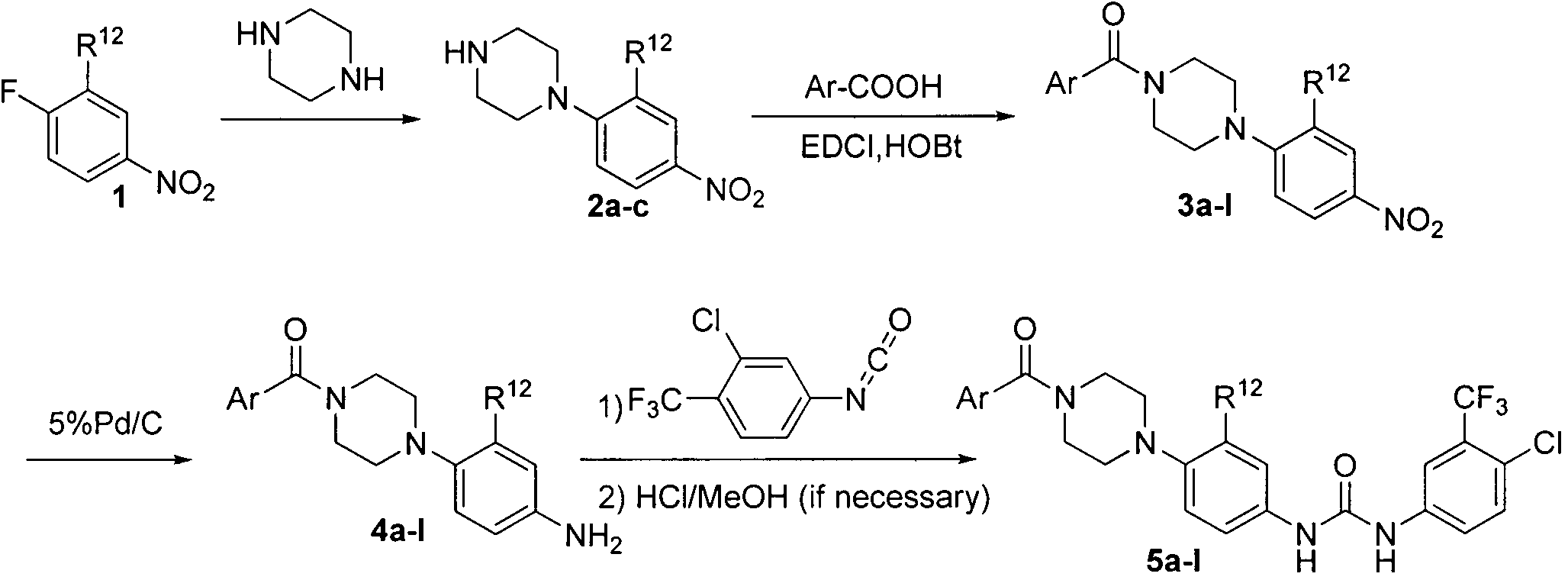
Piperazine structure-based aryl formamide Raf kinase inhibitor and preparation method as well as application thereof
A piperazine, phenyl technology, applied in the field of medicinal chemistry, can solve the problems of uncontrollable growth and proliferation of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] 1-(4-nitrophenyl)piperazine 2a
[0081] The starting material p-fluoronitrobenzene (4.20g, 30mmol), piperazine (8.61g, 10mmol), potassium carbonate (4.15g, 30mmol) and anhydrous acetonitrile 100ml were added to a 250ml round-bottomed bottle, at 90°C React for 5 hours, after cooling, concentrate under reduced pressure to remove acetonitrile, add about 200ml of water, then transfer to a separatory funnel, extract with ethyl acetate, 100ml×2, wash the organic phase with water, collect the organic phase and dry it, then concentrate by suction filtration After removal of ethyl acetate, it was poured into water to obtain light yellow solid 2a (4.00 g, 19.3 mmol), yield 64.4%. mp: 128~131℃; MS[M+H] + 208.0.
Embodiment 2
[0083] 1-(2-methyl-4-nitrophenyl)piperazine 2b
[0084] Using 1-fluoro-2-methyl-4-nitrobenzene as the starting material, the synthesis method of compound 2a was similar to that of compound 2a to obtain light yellow solid 2b with a yield of 50.3%. MS [M+H] 222.1.
Embodiment 3
[0086] 1-(2-Chloro4-nitrophenyl)piperazine 2c
[0087] Using 1-fluoro-2-chloro-4-nitrobenzene as the starting material, similar to the synthesis method of compound 2a, a light yellow solid 2c was obtained with a yield of 61.67%. MS [M+H] 242.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com