Tablet-type composition for oral administration and method for producing same
A manufacturing method and composition technology, which is applied in the field of tablet-type oral administration adsorbent composition, can solve the problems of inability to take fine granules, capsules, large doses of capsules, and great pain, and achieve Improved wearability, excellent abrasion resistance, volume reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
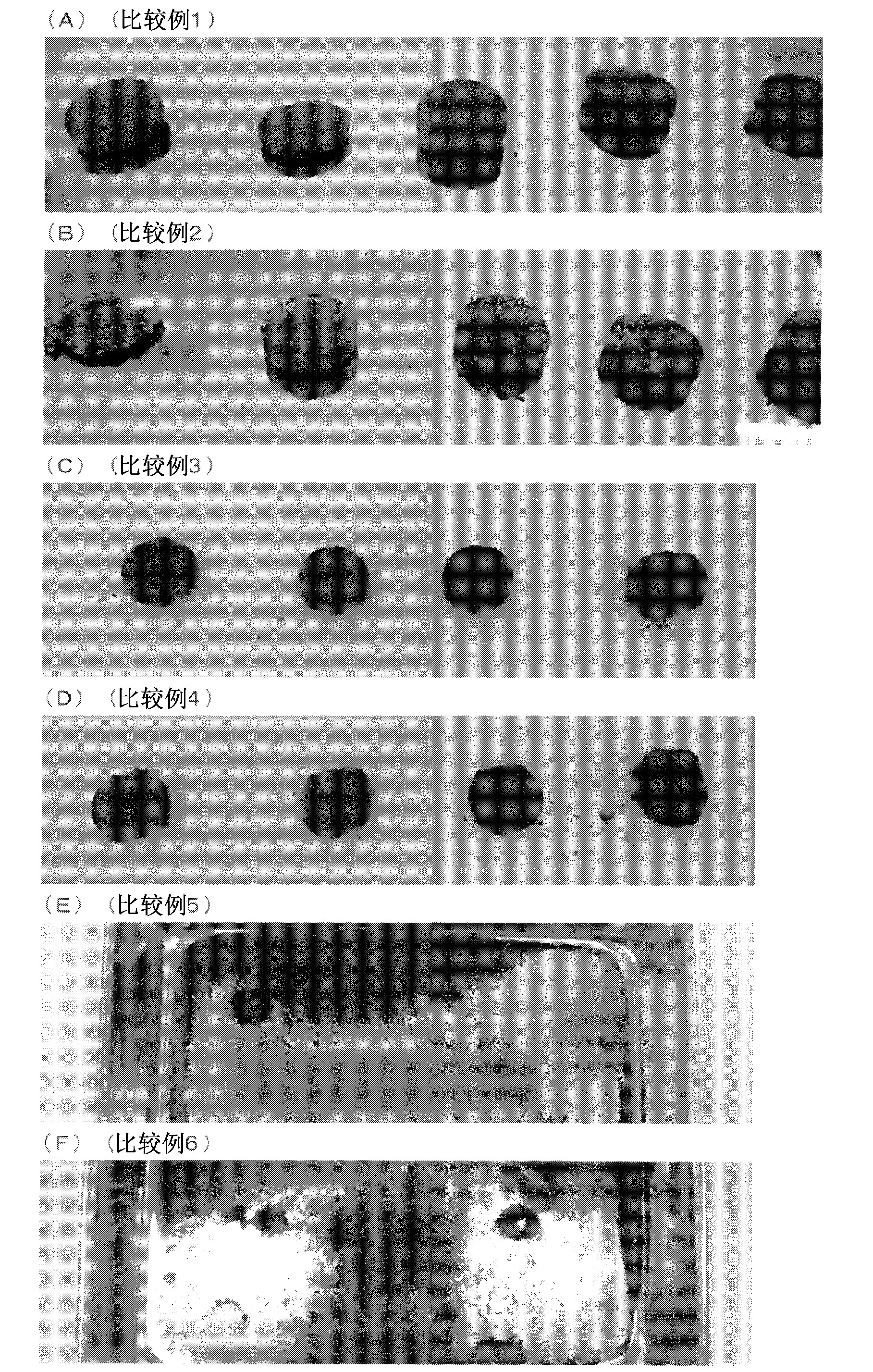
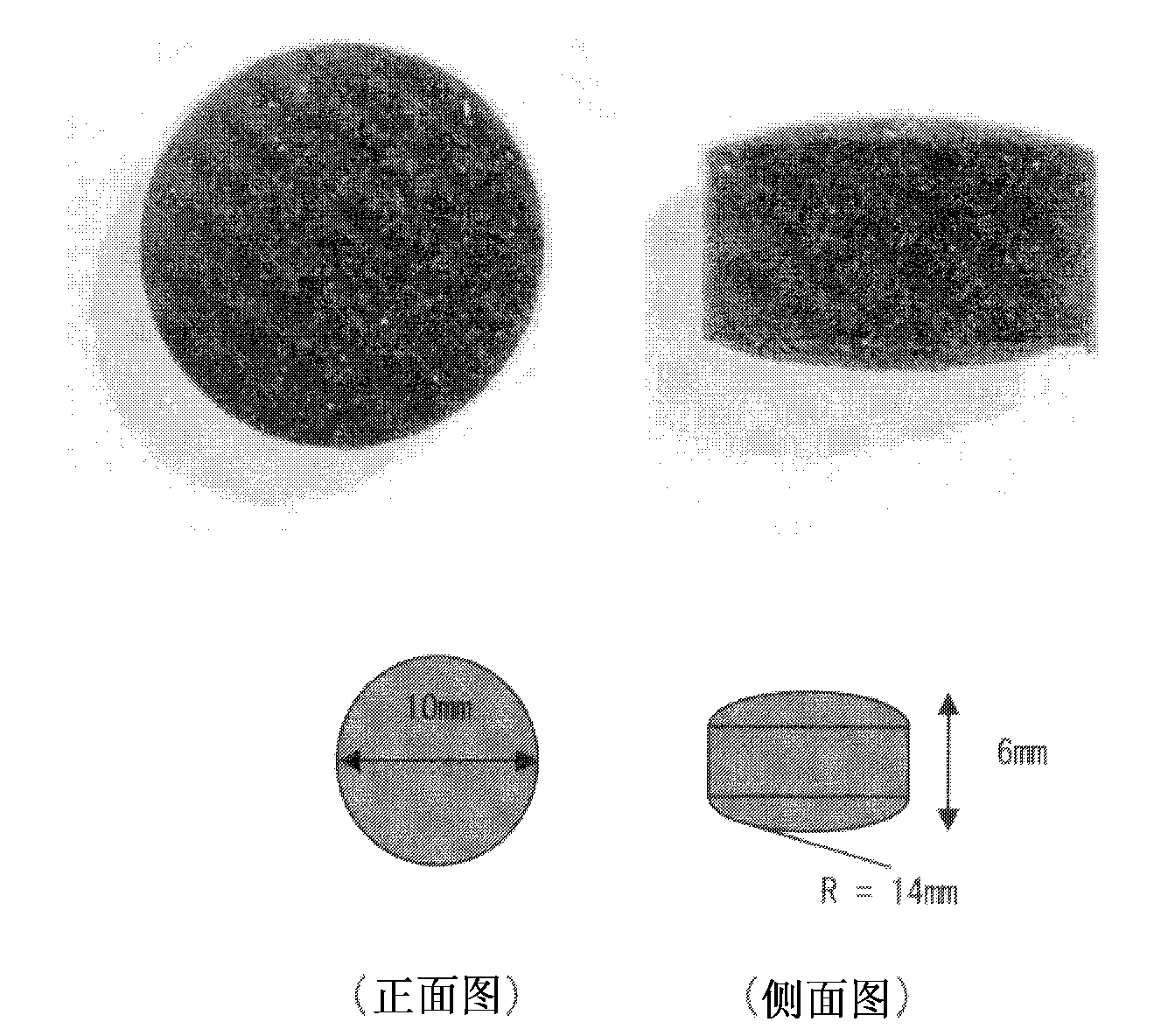
[0261] Hereinafter, although an Example demonstrates this invention concretely, these do not limit the scope of the present invention.
[0262] In this example, the bulk volume, bulk density, hardness of the tablet composition, wear degree of the tablet composition, and tablet composition The sphericity ratio of the spherical activated carbon and the indole adsorption speed test were measured by the following methods. In addition, the following measuring method is a measuring method using spherical activated carbon, and it is applicable also to the particulate material used for this invention other than an indole adsorption test.
[0263] (1) Tap density
[0264] Fill a 50mL graduated cylinder with 20g of a sample of spherical activated carbon, tap it 50 times, divide the sample weight by the volume, and calculate the tap density. It should be noted that there is no difference at all between the measured value obtained by this method and the measured value obtained by the pa...
manufacture example 1
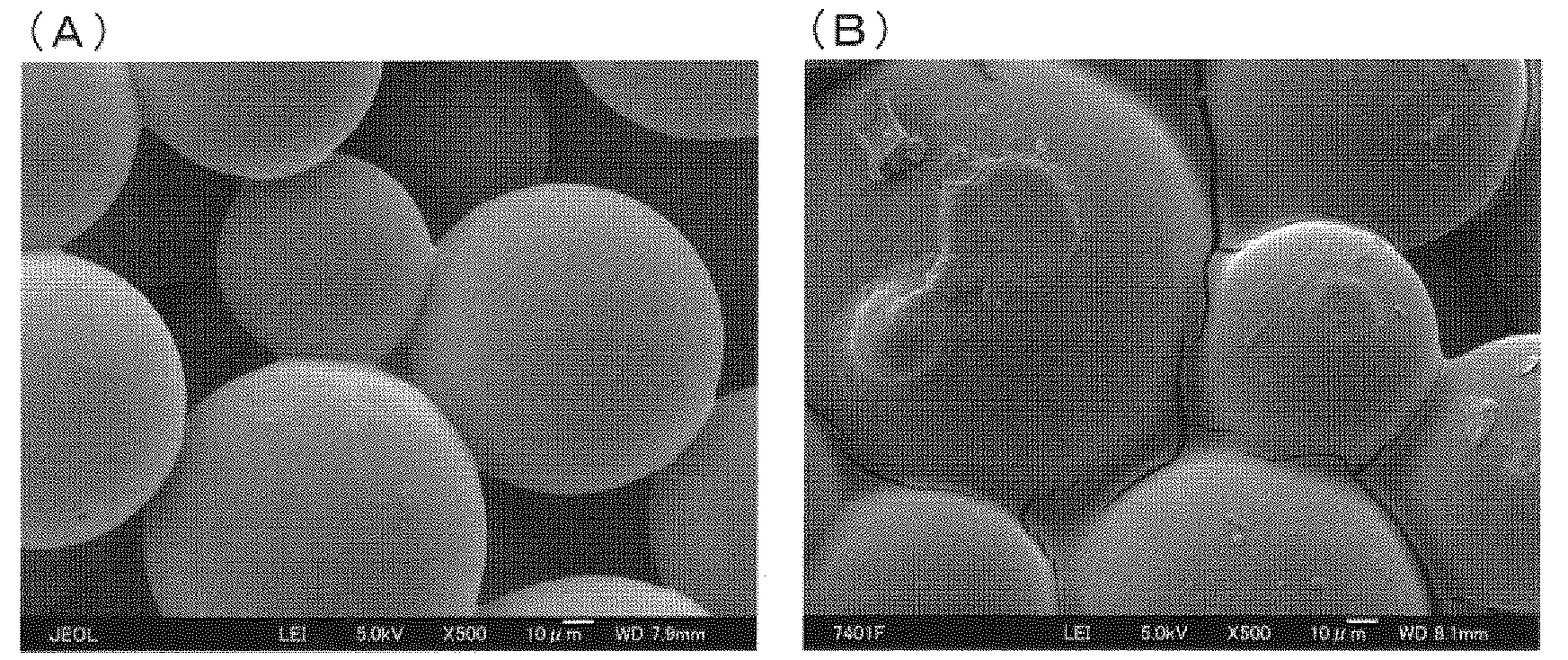
[0313] "Production Example 1: Production of Porous Spherical Carbonaceous Material"
[0314] A porous spherical carbonaceous material was obtained in the same manner as the method described in Example 1 of Patent No. 3522708 (Japanese Patent Laid-Open No. 2002-308785). The specific operation is as follows.
[0315] 68 kg of petroleum-based pitch (softening point = 210°C; quinoline insoluble ratio = 1% by weight or less; H / C atomic ratio = 0.63) and 32 kg of naphthalene were added to a pressure-resistant container with an internal volume of 300 L with stirring blades , after melt-mixing at 180°C, cooling to 80-90°C for extrusion to obtain a strip-shaped molded body. Next, the belt-shaped molded body is crushed so that the ratio of the diameter to the length becomes approximately 1-2.
[0316] In an aqueous solution in which 0.23% by weight of polyvinyl alcohol (saponification degree = 88%) was dissolved and heated to 93°C, the crushed product was added, stirred and dispersed ...
manufacture example 2
[0328] "Production Example 2: Production of Porous Spherical Carbonaceous Material"
[0329]In the same manner as the method described in Example 1 of JP-A-2005-314416, a porous spherical carbonaceous material (surface-modified spherical activated carbon) was obtained. The specific operation is as follows.
[0330] Add 220 g of deionized water and 58 g of methyl cellulose into a 1 L detachable flask, and appropriately add 105 g of styrene and divinylbenzene with a purity of 57% (57% of divinylbenzene and 43% of ethyl Vinylbenzene) 184g, 2,2'-azobis(2,4-dimethylvaleronitrile) 1.68g, and 1-butanol 63g as a porogen, and then replace the system with nitrogen , the two-phase system was stirred at 200 rpm, heated to 55° C., and kept in this state for 20 hours. The resulting resin was filtered, dried with a rotary evaporator, and 1-butanol was distilled off from the resin with a vacuum drier, and then dried under reduced pressure at 90°C for 12 hours to obtain a spherical porous co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com