Method for testing influence of appearance of lithium battery on heat conduction of lithium battery
A lithium battery and heat conduction technology, applied in the field of power supply, can solve problems affecting the performance of lithium batteries
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] In order to make the technical means, creative features, objectives and effects achieved by the present invention easy to understand, the present invention will be further described below in conjunction with specific embodiments.
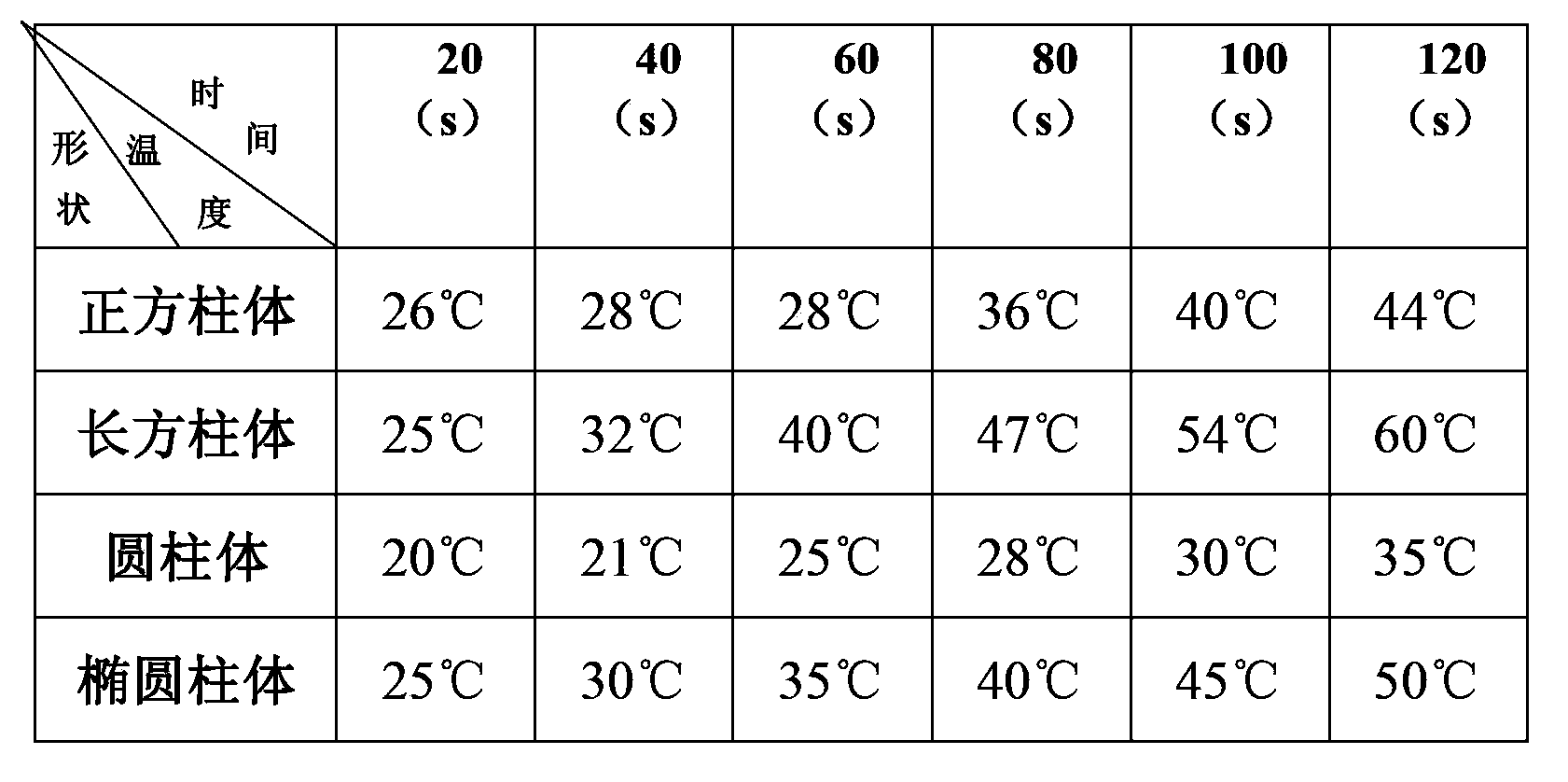
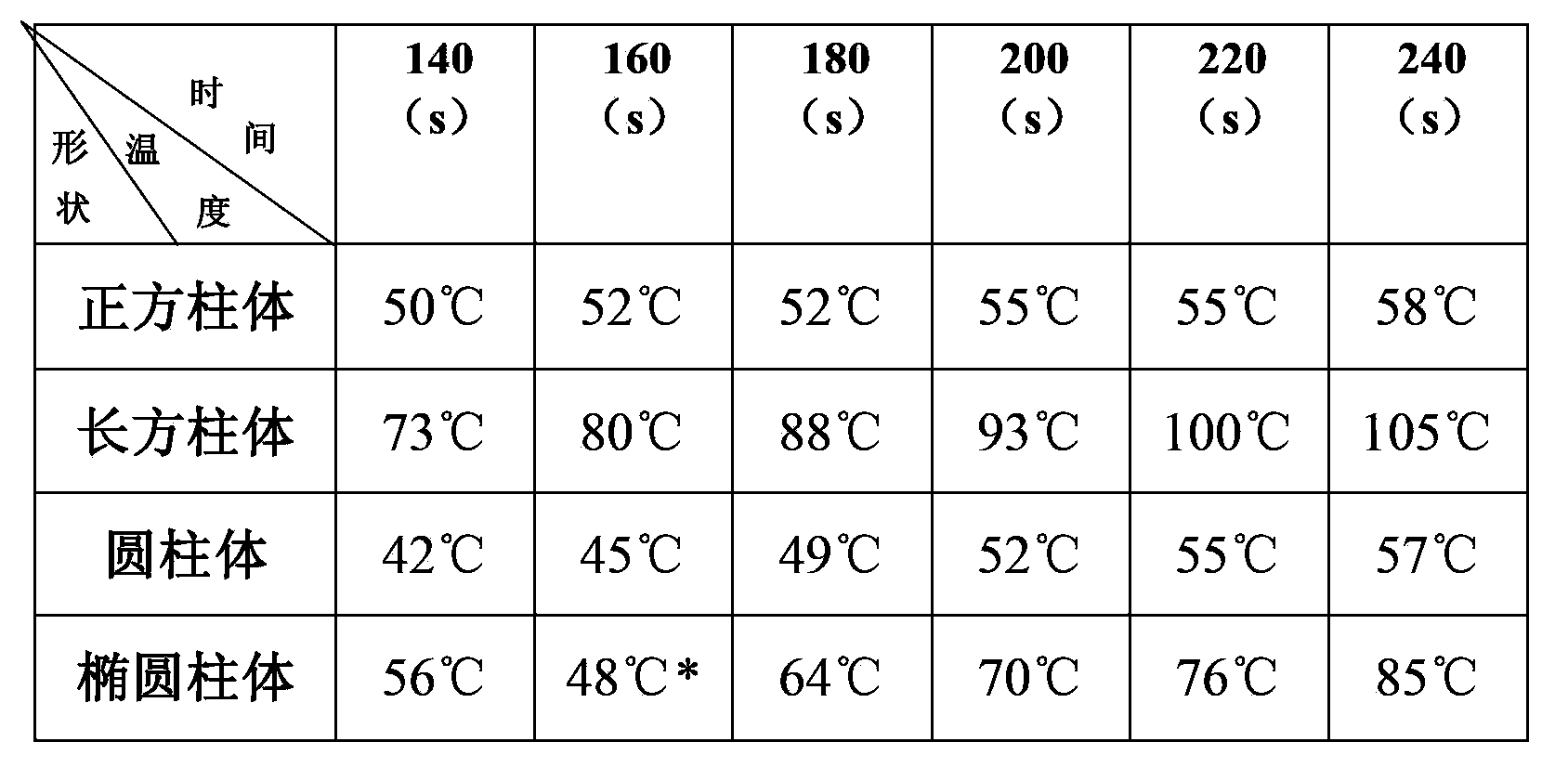
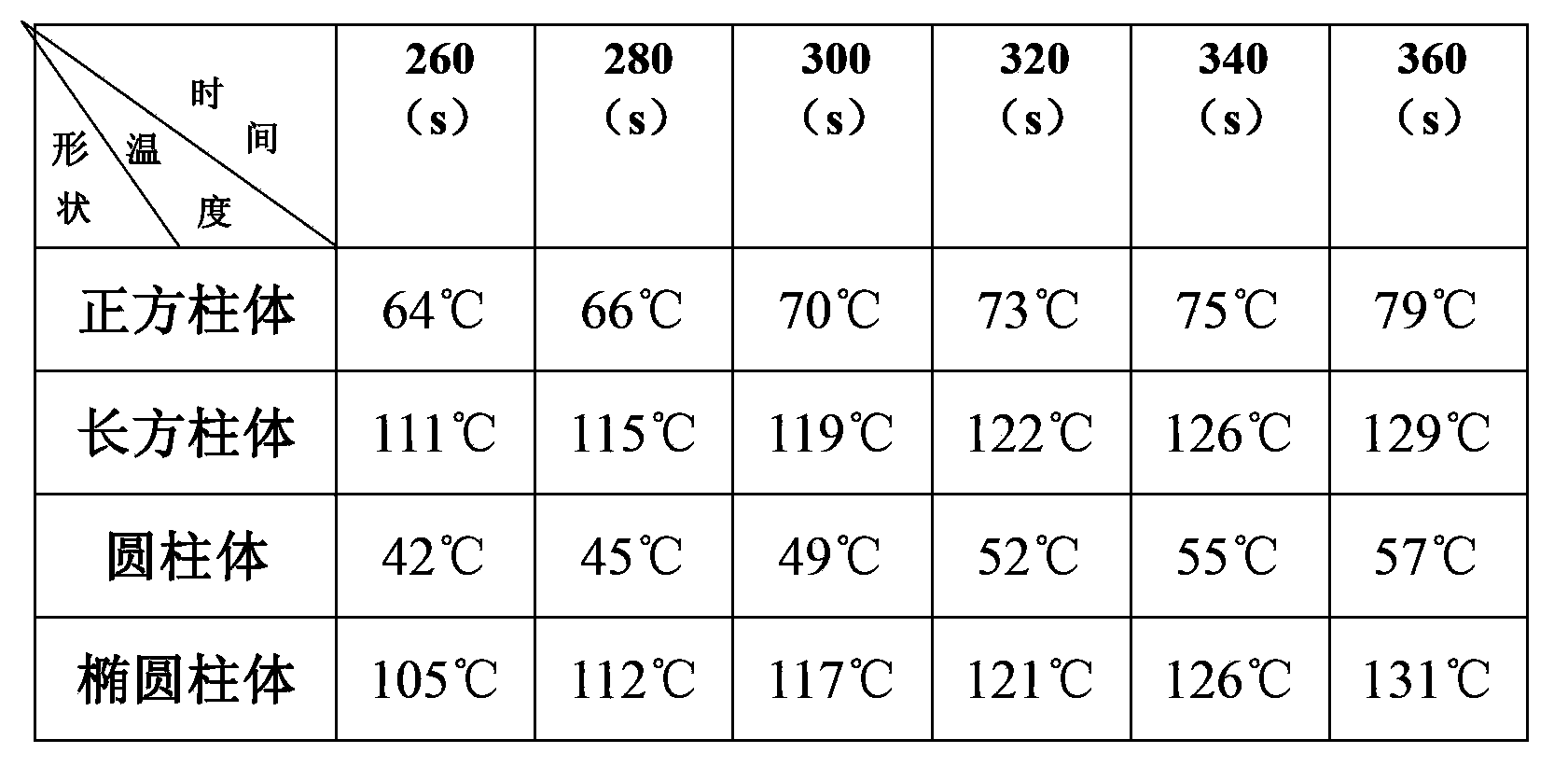
[0024] First of all, the current mainstream research direction of high-temperature lithium batteries is to find new electrolytes, such as molten salt electrolytes that can be liquid at lower temperatures, and to find molten salt electrolytes with higher conductivity. However, changing the shape of the shell of the high-temperature lithium battery has been neglected, and the performance of the high-temperature lithium battery can also be improved by changing the shape of the battery shell of the high-temperature lithium battery.
[0025] Secondly, by changing the shape of the high-temperature lithium battery, the performance of the high-temperature lithium battery can be improved to a certain extent in a relatively quick way, so that it can wor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com