A method for the separation and determination of moxifloxacin hydrochloride and its enantiomers
A technology for moxifloxacin hydrochloride and enantiomers, which is applied in the field of separation and determination of moxifloxacin hydrochloride and its enantiomers, can solve the threat to the safety of clinical medication and the unspecified enantiomer check items and limits and other issues, to achieve the effect of low cost, strong specificity and accurate detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
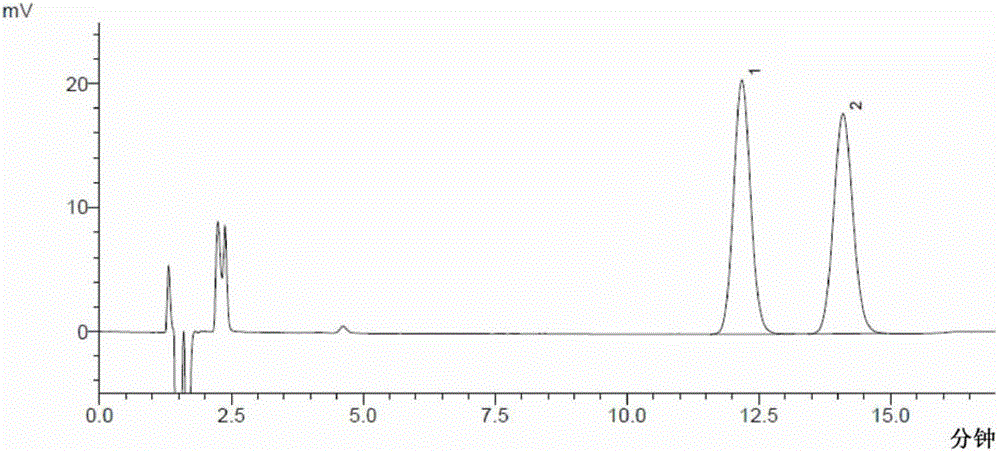
[0095] The determination of embodiment 1 moxifloxacin hydrochloride and its enantiomer
[0096] Chromatographic conditions Shimadzu LC-2010AHT high performance liquid chromatograph, LCsolution workstation, use octylsilane bonded silica gel as filler, use copper sulfate D-phenylalanine solution (the moles of copper sulfate and D-phenylalanine The ratio is 1:2, add water to dissolve and prepare copper sulfate concentration of 4mmol / L, adjust pH to 4.0)-methanol (75:25) as mobile phase, column temperature: 35°C; flow rate: 1.0ml / min; detection The wavelength is: 293nm.
[0097] Sample preparation Take about 30mg of moxifloxacin hydrochloride, put it in a 50ml measuring bottle, add mobile phase to dissolve and dilute to the mark, shake well, and use it as the test solution; take another about 15mg of moxifloxacin hydrochloride racemate, put it in a 100ml measuring bottle Add mobile phase to dissolve and dilute to the mark, shake well, take 1ml, put it in a 10ml measuring bottle, ...
Embodiment 2
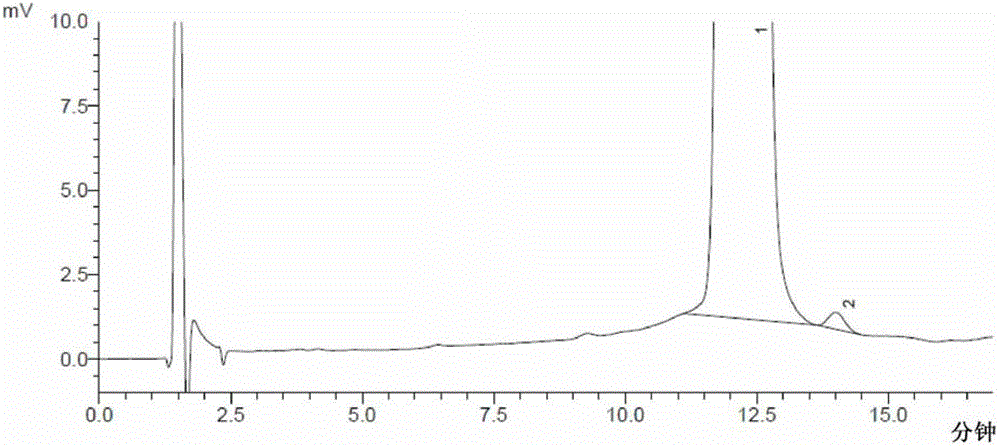
[0103] The determination of embodiment 2 moxifloxacin hydrochloride and its enantiomer
[0104] Chromatographic conditions Shimadzu LC-2010AHT high performance liquid chromatography, LCsolution workstation, using octadecylsilane bonded silica gel as filler, copper sulfate L-phenylalanine solution (copper sulfate and L-phenylalanine The molar ratio is 1:4.5, add water to dissolve and prepare copper sulfate concentration of 3mmol / L, adjust pH to 4.5)-methanol (79:21) as mobile phase, column temperature: 30°C; flow rate: 0.8ml / min; The detection wavelength is: 293nm.
[0105] Sample preparation Take about 35mg of moxifloxacin hydrochloride, put it in a 50ml measuring bottle, add mobile phase to dissolve and dilute to the mark, shake well, and use it as the test solution; take another about 20mg of moxifloxacin hydrochloride racemate, put it in a 100ml measuring bottle Add mobile phase to dissolve and dilute to the mark, shake well, take 1ml, put it in a 100ml measuring bottle, a...
Embodiment 3
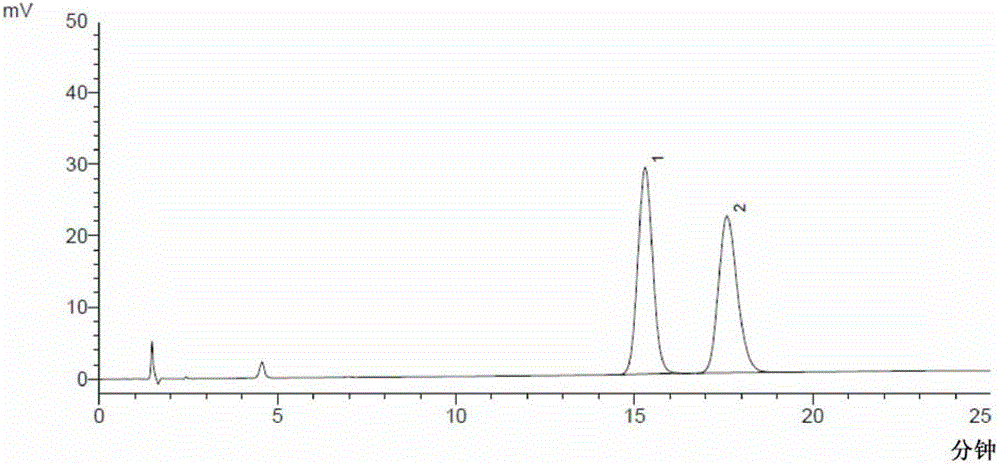
[0111] The determination of embodiment 3 moxifloxacin hydrochloride and its enantiomer
[0112]Chromatographic conditions Shimadzu LC-2010AHT high performance liquid chromatograph, LCsolution workstation, using octadecylsilane bonded silica gel as filler, copper sulfate L-isoleucine solution (copper sulfate and L-isoleucine The molar ratio is 1:5.5, add water to dissolve and prepare copper sulfate concentration of 2mmol / L, adjust pH to 3.5)-methanol (77:23) as mobile phase, column temperature: 40°C; flow rate: 1.0ml / min; The detection wavelength is: 293nm.
[0113] Sample preparation Take about 45mg of moxifloxacin hydrochloride, put it in a 100ml measuring bottle, add mobile phase to dissolve and dilute to the mark, shake well, and use it as the test solution; take another about 13mg of moxifloxacin hydrochloride racemate, put it in a 100ml measuring bottle Add mobile phase to dissolve and dilute to the mark, shake well, take 1ml, put it in a 10ml measuring bottle, add mobil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com