Protopanoxadiol derivative, preparation method of derivative, composition containing derivative and application of composition
A technology of protopanaxadiol and derivatives, which can be used in drug combinations, steroids, pharmaceutical formulations, etc., can solve the problems of inability to achieve reversal concentration, changing pharmacokinetic parameters of antitumor drugs, and toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
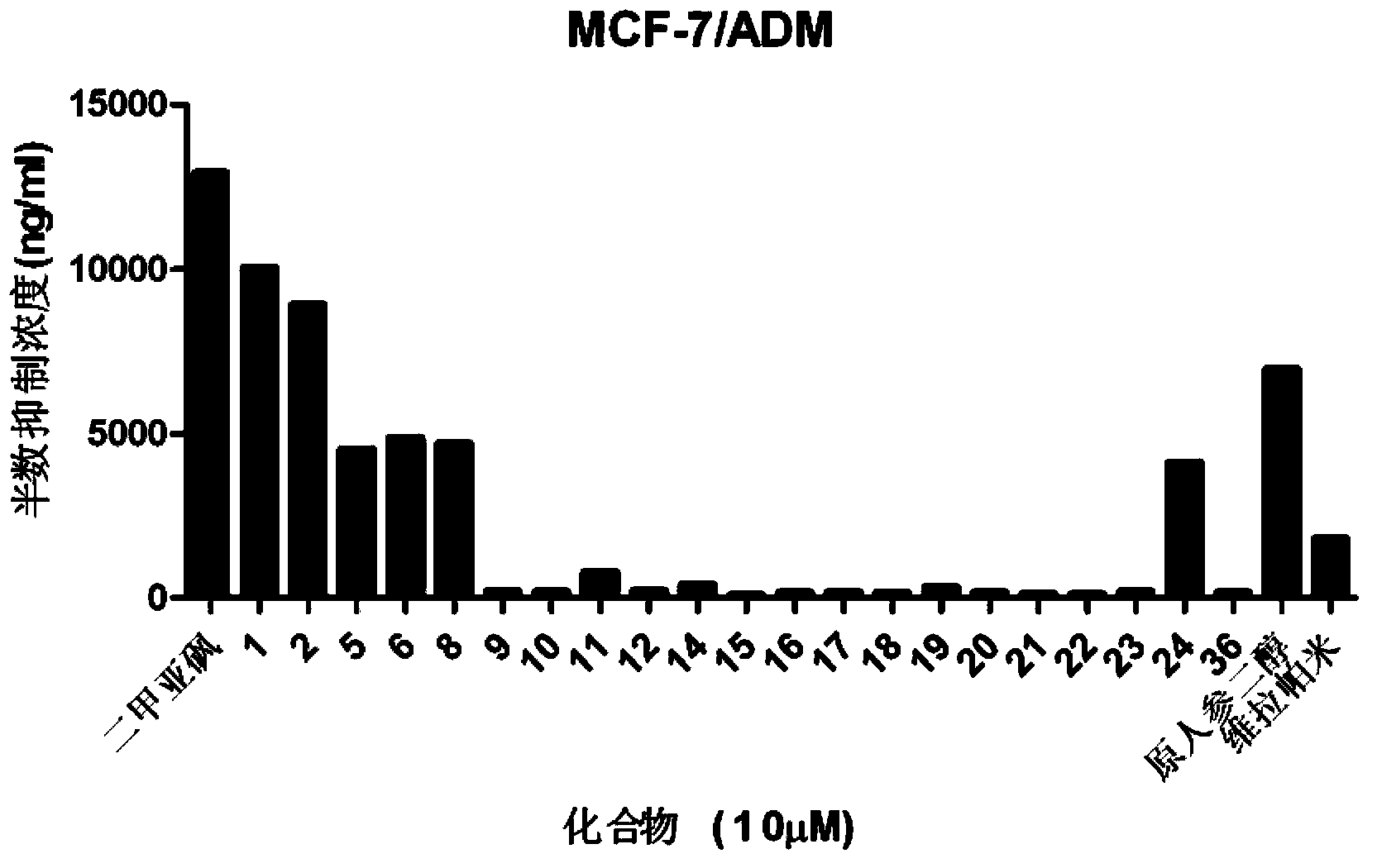
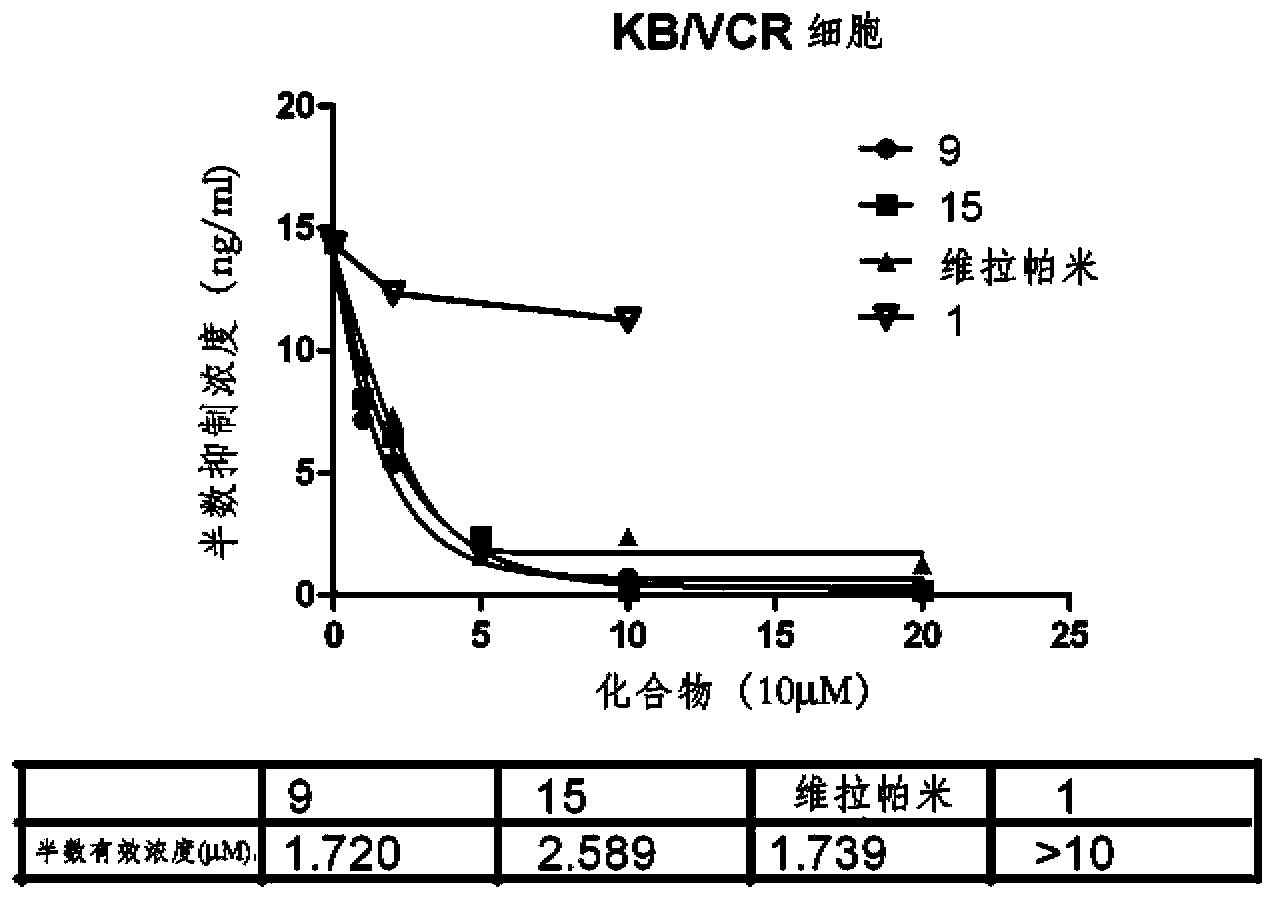
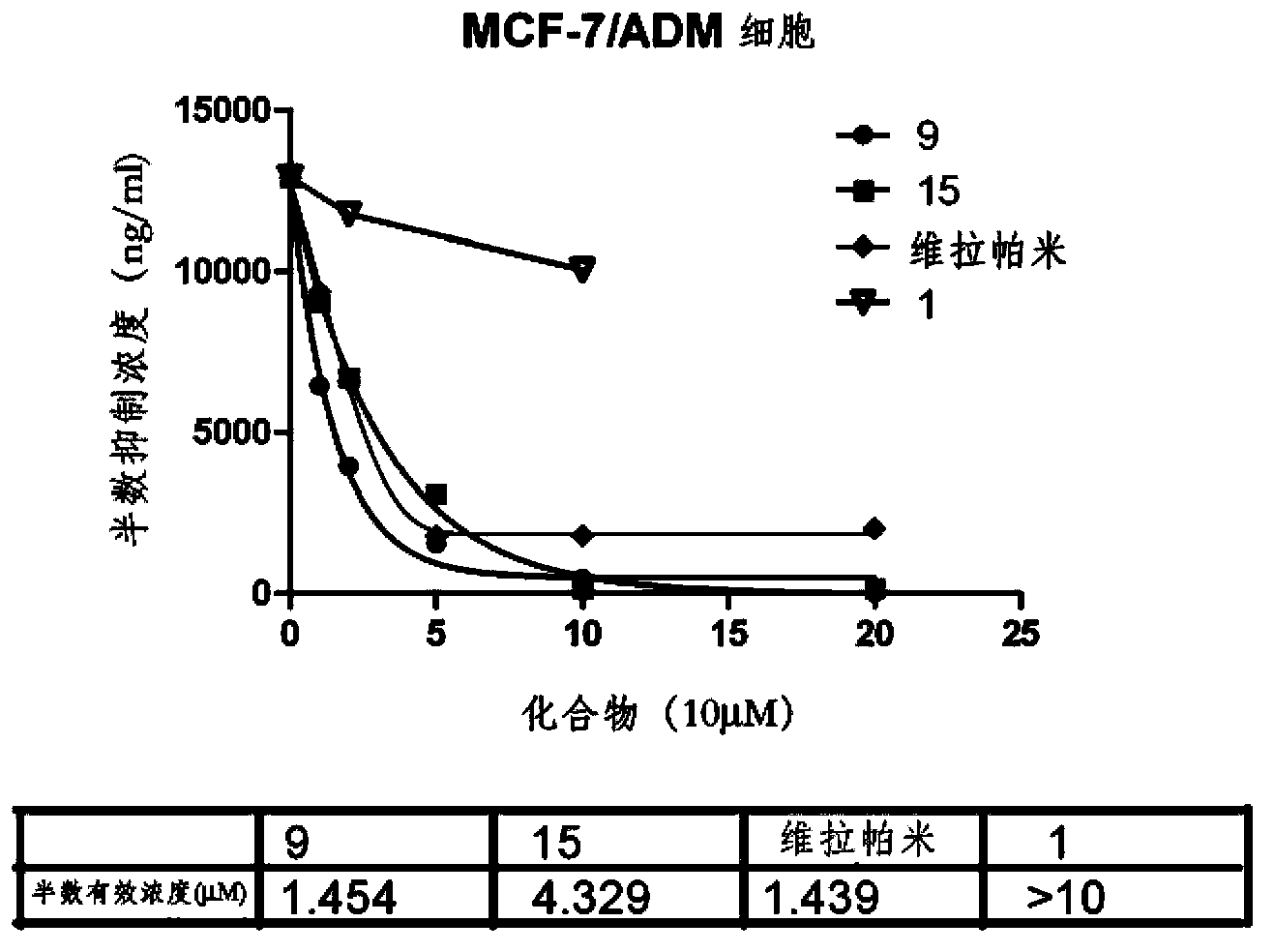
Image
Examples
preparation example Construction
[0061] Preparation of compound 1-26:
[0062] The starting materials used in the preparation of the following compounds are protopanaxadiol (PPD), which is obtained by synergistic oxidation of total ginsenosides with oxygen and tert-butanol peroxide [CN200510100735]. As shown in Reaction Formula 1, at -78°C, the double bond of compound PPD is broken by ozone, and then amine and NaBH(OAc) are added at -3°C 3 , to obtain the target compound by reductive amination.
[0063]
[0064] Reaction 1
Embodiment 1
[0065] The preparation of embodiment 1 compound 1
[0066]
[0067] In a 50ml three-neck reaction flask, dissolve PPD (200mg, 0.435mmol) in 15ml CH 2 Cl 2 After stirring for 10 min at -78°C, O 3 Reaction 2min. Then warm up to -3°C, add methylamine (0.1ml), NaBH(OAc) 3 (368.8mg, 1.7mmol) and CH 3 OH (8ml), stir overnight, after the reaction is complete, add 15ml of water, 2 Cl 2 (30ml) extracted twice, dried over anhydrous sodium sulfate, concentrated CH under reduced pressure 2 Cl 2 , after silica gel column chromatography (dichloromethane / methanol / triethylamine, 40:1:0.5), the product 1 was obtained with a yield of 85%. 1 H NMR (300MHz, CDCl 3 )δ4.12(m,1H),3.52(td,J=13.0,6.2Hz,1H),3.21(dd,J=10.9,5.0Hz,1H),2.94(m,1H),2.62(m,1H ),2.47(s,3H),1.13(s,3H),0.99(s,3H),0.98(s,3H),0.88(s,6H),0.78(s,3H),0.73(d,J= 11.0Hz, 1H), other high-field H signals overlap at 1.24-2.10; ESI-MS 450.7[M+H] + .
Embodiment 2
[0068] The preparation of embodiment 2 compound 2
[0069]
[0070] Compound 2 was prepared in the same manner as in Example 1 except that ethylamine was used instead of methylamine; the yield was 86% by silica gel column chromatography (dichloromethane / methanol / triethylamine, 45:1:0.5). 1 H NMR (300MHz, CDCl 3 )δ3.55(td,J=12.9,6.3Hz,1H),3.19(dd,J=10.9,4.9Hz,1H),2.93(m,1H),2.72(m,1H),2.58(m,1H ),2.48(m,1H),2.09(m,1H),1.15(s,3H),1.11(t,J=7.2Hz,3H),0.99(s,3H),0.98(s,3H),0.89 (s,3H),0.88(s,3H),0.78(s,3H),0.73(d,J=10.7Hz,1H), other high-field H signals overlapped at 1.02-2.10; ESI-MS464.7[M +H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com