A novel glycosyltransferase derived from dolwoe and use thereof
A technology of glycosyltransferase and glucose, applied in the direction of glycosyltransferase, transferase, enzyme, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Cloning and purification of UDP-glycosyltransferase GpUGT23 derived from Gynostemma pentaphyllum
[0058] Gene amplification from Gynostemmapentaphyllum cDNA by PCR using GpUGT23-F (5'-GATCGGATCCATGAAGAAAATTTTGATGTTTCC-3'; Sequence 3), GpUGT23-R (5'-GATCCTCGAGTATTTTTGCTTGACAAAGC-3'; Sequence 4) primers and polymerase , each end of the gene was cut with BamHI and XhoI as restriction enzymes. Then, it was cloned into pET50 (Ohetal. PlantCell, 16, 3045-3058.2004) vector to prepare an expression vector, and the expression vector was transformed into Escherichia coli BL21(DE3)-RIL strain to prepare a GpUGT23 expression strain. The protein was obtained after the expression of the protein was induced by using IPTG, and the GpUGT23 enzyme was purified by Ni-NTAHis-binding resin.
Embodiment 2
[0059] Embodiment 2: Invitro enzyme experiment
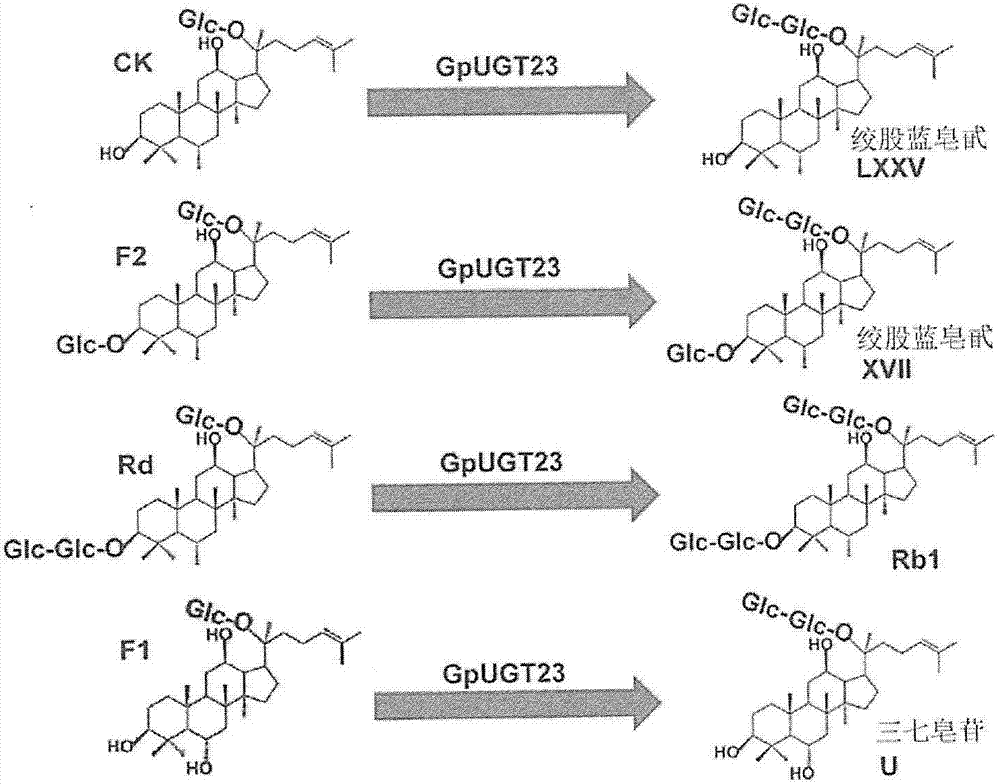
[0060] Glycosyltransferase experiments were performed in a reaction buffer solution (10 mM PBS buffer, pH 7) containing purified GpUGT23 (30 μg), ginsenoside compound (5 mM) and UDP-glucose (50 mM). For this purpose, 4 different kinds of ginsenosides, ie, CK (compound K), F2, Rd and F1 were used to react with the enzyme of the present invention.
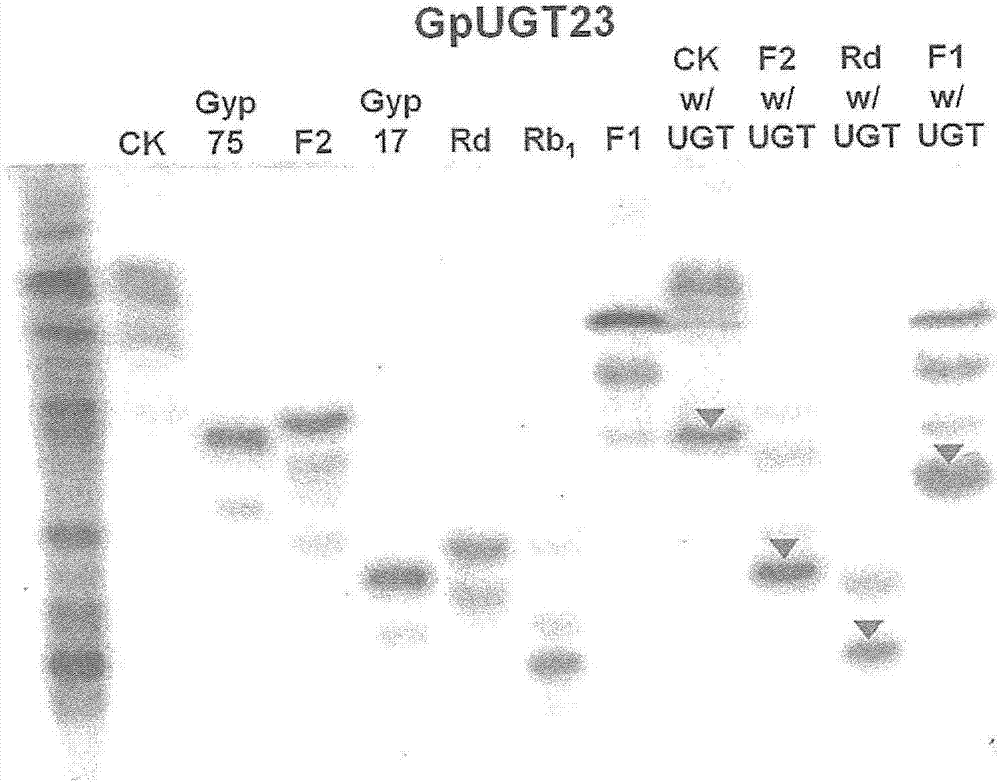
[0061] After the reaction mixture was incubated at 37° C. for 12 hours, the product was analyzed by TLC (thin-layer chromatography) or HPLC (high performance liquid chromatography).
[0062] Use 60F with mobile phase (acetone:methanol:DDW=65:35:10vol / vol) 254 TLC analysis was performed on silica gel plates (Merck, Germany). Using 10% (vol / vol) sulfuric acid (H 2 SO 4 ) to detect by spraying the dissolved product (resolved product) on the TLC plate, and then heating at 110° C. for 5 minutes ( figure 2 ).
[0063] HPLC analysis was performed using an ODS(2) C18 column (Phenomenex, ...
experiment example 1
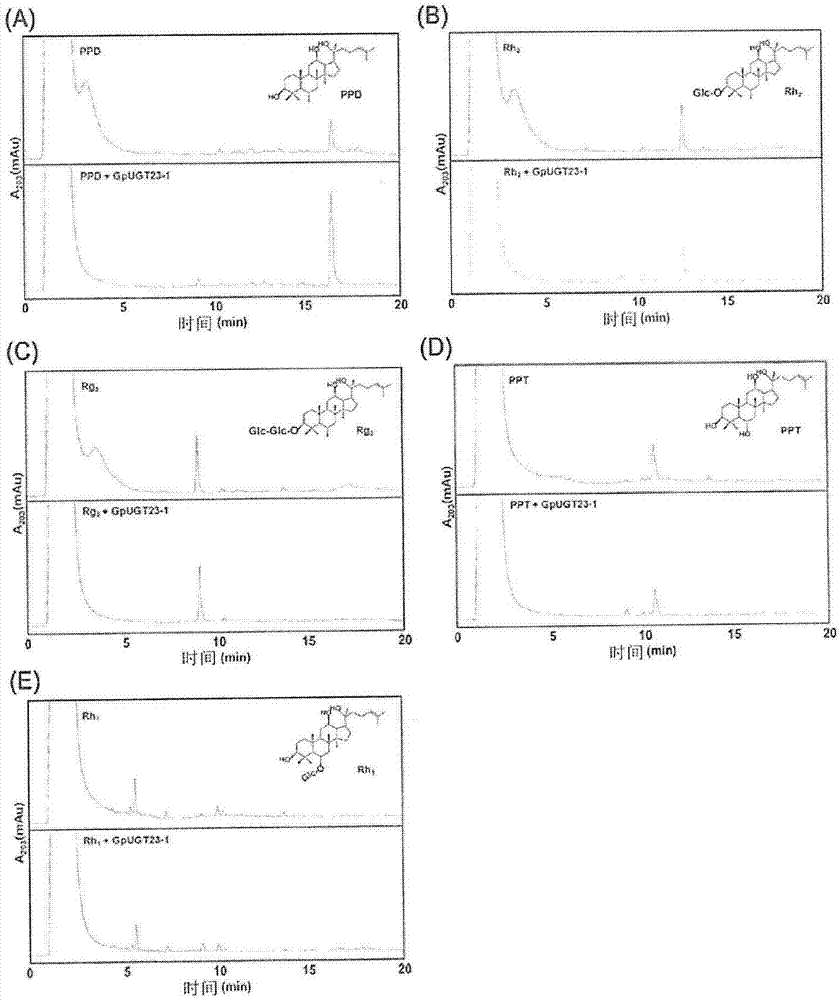
[0065] Experimental Example 1: GpUGT23 is linked to the 20th carbon of PPD and PPT ginsenosides through a glycosidic bond Confirmation of specific glycosyltransferase activity on glucose
[0066] By the method described in Example 2, it was confirmed whether or not GpUGT23 has substrate specificity and regioselectivity.
[0067] First, using 9 kinds of ginsenosides (PPD, Rh 2 , Rg 3 , CK, F2, Rd, PPT, F1 and Rh 1 ), in the presence of UDP-glucose, when GpUGT23 as the recombinant GpUGT of Example 1 was reacted, it was confirmed by TLC (thin-layer chromatography, thin-layer chromatography) that in CK, F2, Rd and F1 reaction.
[0068] In order to confirm this again, four different ginsenosides (CK, F2, Rd, and F1) were co-cultured with GpUGT23 to react, and the product transformed by the recombinant GpUGT23 was analyzed by TLC analysis. show the result in figure 2 . CK, Gyp75 (Gypenoside LXXV), F2, Gyp17 (Gypenoside XVII), Rd, Rb used as standard samples for migrating s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com