Synthesis and preparation process of rgd cyclic peptide
A preparation process and cyclic peptide technology, applied in the field of solid-phase peptide synthesis, can solve the problems of complexity, trouble, and low crude product yield, and achieve the effects of high product yield, reasonable process and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
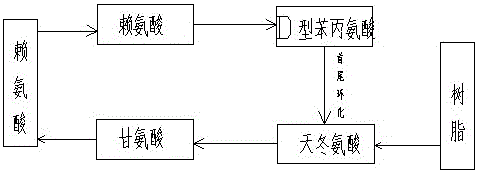
[0018] Synthetic peptide sequence cyclo(RGDfK):
[0019] The cyclic peptides are formed by amide bonds at the beginning and end. Select the company's semi-automatic peptide synthesizer, weigh an appropriate amount of 2-chlorotrityl chloride resin into the reaction glass column, add an appropriate amount of DMF to soak and swell, weigh an appropriate amount of FMOC-Asp(oall)-OH protected amino acid and add it to the resin , add an appropriate amount of DMF solvent as the reaction solution, blow nitrogen to dissolve the amino acid, add an appropriate amount of DIEA catalyst, react at room temperature for 1 hour, then add a small amount of methanol solvent to react for 20 minutes to seal off the reactive sites on the resin. The resin was repeatedly washed with DMF and methanol solvents and the solvent was aspirated so that the first amino acid, aspartic acid, was attached to the resin. An appropriate amount of piperidine solvent was added to react for 15 minutes to remove the am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com