Method for synthesizing multi-substituted silicon-based allene in highly selective way
A silicon-based allene and multi-substitution technology, which is applied in the field of synthesizing multi-substituted silicon-based allenes, can solve the problems of expensive environment, unfriendliness, and poor functional group compatibility, and achieve good functional group compatibility, easy separation and purification, and mild conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
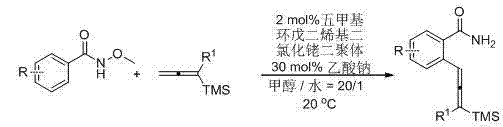
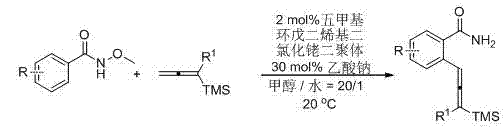
[0018] Add to the reaction tube N -Methoxybenzamide (151.7 mg, 1 mmol), pentamethylcyclopentadienyl rhodium dichloride dimer (12.4 mg, 0.02 mmol), sodium acetate (25 mg, 0.3 mmol), 3- Trimethylsilyl-1,2-heptadiene (25 mg, 0.3 mmol), methanol (6 mL), water (0.3 mL), stirred at room temperature, and reacted for 11 h. Filter the short column with ether, concentrate, and obtain by flash column chromatography. 261.1 mg of 2-(3-trimethylsilyl-1,2-heptadien-1-yl)benzamide was obtained as a solid, with a yield of 91%.
[0019] Melting point 93.2-94.4 o C (hexane / ethyl acetate); 1 H NMR (300 MHz, CDCl 3 ) δ 7.51 (t, J = 7.7 Hz, 2 H, Ar-H), 7.37 (t, J = 7.7 Hz, 1 H, Ar-H), 7.27 (brs, 1 H, one proton of NH 2 ), 7.16 (t, J = 7.4 Hz, 1 H, Ar-H), 6.52 (t, J = 2.9 Hz, 1 H, CH=), 6.16 (brs, 1 H, one proton of NH 2 ), 2.27-2.03 (m, 2 H, CH 2 ), 1.66-1.24 (m, 4 H, CH 2 × 2), 0.93 (t, J = 7.2 Hz, 3 H, CH 3 ), 0.20 (s, 9 H, TMS); 13 C NMR (75 MHz, CDCl 3 ) δ 205.5, 172.4, 1...
Embodiment 2
[0021] By the method described in Example 1, the difference is that the substrate and reagent used are: 4-chloro- N -Methoxybenzamide (1.8570g, 10 mmol), pentamethylcyclopentadienyl rhodium dichloride dimer (124.2 mg, 0.2 mmol), sodium acetate (246.7 mg, 3 mmol), 3- Trimethylsilyl-1,2-heptadiene (1.6789 g, 10 mmol), methanol (60 mL), water (3 mL), reacted at room temperature for 16 h to give the product 4-chloro-2-(3-tri Methylsilyl-1,2-heptadien-1-yl)benzamide 2.5643 g, solid, yield 80%.
[0022] Melting point 85.6-85.9 o C (hexane / ethyl acetate); 1 H NMR (300 MHz, CDCl 3 ) δ 7.46-7.34 (m, 2 H, Ar-H), 7.10 (dd, J 1 = 8.4 Hz, J 2 = 1.5 Hz, 1 H, Ar-H), 6.75 (brs, 1 H, one proton of NH 2 ), 6.40 (t, J = 1.8 Hz, 1 H, CH=), 5.96 (brs, 1 H, one proton of NH 2 ), 2.26-2.01 (m, 2 H, CH 2 ), 1.62-1.22 (m, 4 H, CH 2 × 2), 0.89 (t, J = 7.2 Hz, 3 H, CH 3 ), 0.16 (s, 9 H, TMS); 13 C NMR (75 MHz, CDCl 3 ) δ 205.2, 171.4, 136.7, 136.3, 130.7, 129.1, 126.4, 125.5, 101.5...
Embodiment 3
[0024] According to the method described in Example 1, the difference is that the substrate and reagent used are: 4-methoxycarbonyl- N -Methoxybenzamide (209.5 mg, 1 mmol), pentamethylcyclopentadienyl rhodium dichloride dimer (12.6 mg, 0.02 mmol), sodium acetate (25.5 mg, 0.3 mmol), 3- Trimethylsilyl-1,2-heptadiene (168.2 mg, 1 mmol), methanol (6 mL), water (0.3 mL), reacted at room temperature for 14 h to give the product 4-methoxycarbonyl-2-(3 - Trimethylsilyl-1,2-heptadien-1-yl) benzamide 297.4 mg, liquid, yield 86%.
[0025] 1 H NMR (300 MHz, CDCl 3 ) δ 8.14 (d, J = 1.2 Hz, 1 H, Ar-H), 7.77 (dd, J 1 = 8.1 Hz, J 2 = 1.2 Hz, 1 H, Ar-H), 7.51 (d, J = 7.8 Hz, 1 H, Ar-H), 6.51 (brs, 1 H, one proton of NH 2 ), 6.39 (t, J = 2.9 Hz, 1 H, CH=), 5.97 (brs, 1 H, one proton of NH 2 ), 3.92 (s, 3 H, OCH 3 ), 2.27-2.01 (m, 2 H, CH 2 ), 1.62-1.22 (m, 4 H, CH 2 × 2), 0.88 (t, J = 7.2 Hz, 3 H, CH 3 ), 0.18 (s, 9 H, TMS); 13 C NMR (75 MHz, CDCl 3 ) δ 205.5, 171.1, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap