Method for preparing pharmaceutical composition containing repaglinide and metformin hydrochloride
A technology for metformin hydrochloride and metformin, applied in the field of medicine, can solve the problems of large specification of metformin hydrochloride, difficult to guarantee uniformity, small specification of repaglinide, etc., and achieves the effects of good fluidity, poor compressibility improvement, and stable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 (1000 pieces amount)
[0019]
[0020] Preparation:
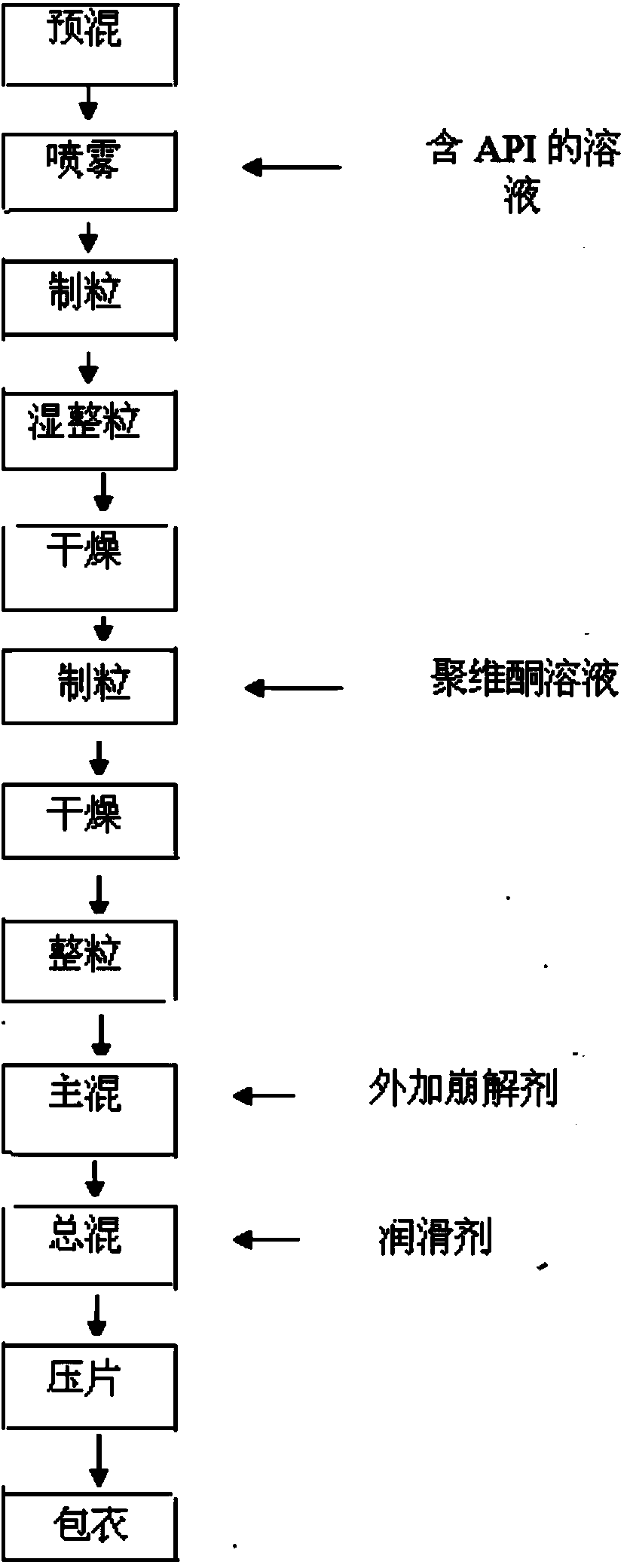
[0021] 1) Dissolving repaglinide, meglumine, and poloxamer in ethanol solution to obtain a mixed solution;
[0022] 2) Grind metformin hydrochloride and sieve it for later use;
[0023] 3) Weigh the formulated amount of metformin hydrochloride, microcrystalline cellulose PH101, crospovidone XL-10 and polyethylene glycol 6000, and place them in a high-shear mixing granulator to mix evenly;
[0024] 4) Add the mixed solution obtained in step 1) into a high-shear granulator, stir evenly to make soft material, pass through a 2mm screen and use a lifting granulator to granulate, mix evenly and place it in a fluidized bed to dry, set the material temperature 60°C;
[0025] 5) Spray the prepared povidone K30 solution into the fluidized bed, set the parameters of atomization pressure and spray speed for one-step granulation, and set the material temperature to 60°C;
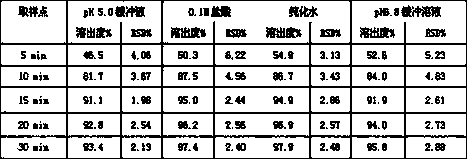
[0026] 6) Mix the prepared granules w...
Embodiment 2
[0028] Example 2 (quantity of 1000 tablets, unit: g)
[0029]
[0030] Preparation:
[0031] 1) Dissolving repaglinide, meglumine, and poloxamer in ethanol solution to obtain a mixed solution;
[0032] 2) Grind metformin hydrochloride and sieve it for later use;
[0033] 3) Weigh the formulated amount of metformin hydrochloride, microcrystalline cellulose PH101, crospovidone XL-10 and polyethylene glycol 6000, and place them in a high-shear mixing granulator to mix evenly;
[0034] 4) Add the mixed solution obtained in step 1) into a high-shear granulator, stir evenly to make soft material, pass through a 2mm screen and use a lifting granulator to granulate, mix evenly and place it in a fluidized bed to dry, set the material temperature 60°C;
[0035] 5) Spray the prepared povidone K30 solution into the fluidized bed, set the parameters of atomization pressure and spray speed for one-step granulation, and set the material temperature to 60°C;
[0036] 6) Mix the prepare...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap