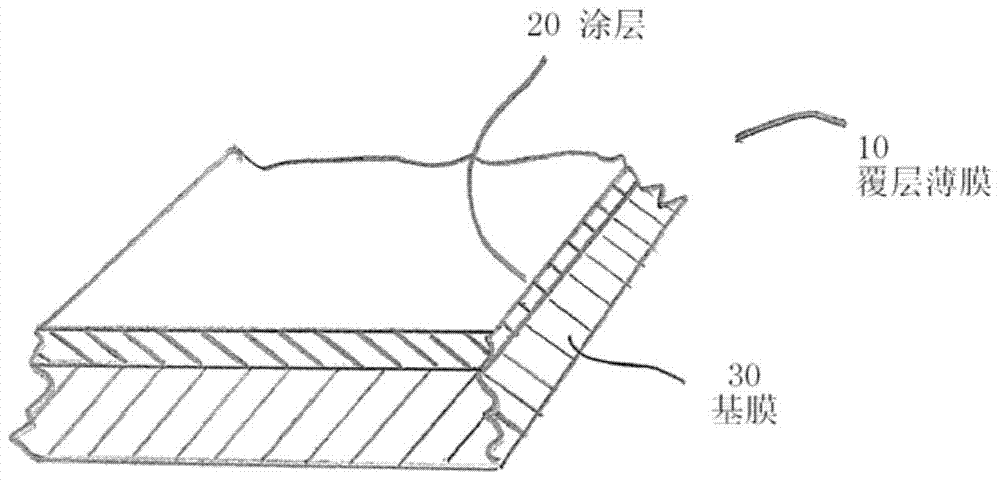
Barrier coatings for thin films and structures
A film and coating technology, applied in coating, thin material handling, transportation and packaging, etc., can solve the problem of difficulty in maintaining vermiculite particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] A 6.5% polyvinyl alcohol (PVOH) batch was prepared by dissolving 193.6 kg of Elvanol 70-62 (Elvanol is a registered trademark of DuPont) in 2914.5 kg of deionized water at 80°C. The PVOH was added to the preheated water with vigorous stirring during the addition. Methylparaben was added to the mixture at 0.3% by weight of Elvanol plus water or 9 kg. The solution was stirred and heated overnight before all the PVOH and methylparaben dissolved. The solution was then cooled and filtered through cheesecloth supported on a stainless steel sieve to remove solid impurities. Check the concentration using a hand-held refractometer and adjust to account for water evaporated during the dissolution step. The evaporated water was replaced with deionized water so that the refractometer read 7.5 BRIX (based on the known relationship between BRIX readings and concentration, a reading of 7.5 BRIX corresponds to a PVOH solids concentration of 6.5% in water).
[0126] Prepare the solut...
Embodiment 2
[0129] Prepare the solution in a 10-ounce plastic cup. Place 26.1 grams of deionized water in the cup. An additional 2.9 grams of isopropanol was added and the contents mixed. To this mixture was added 0.3 grams of lithium hydroxide and the contents mixed until the particles were completely dissolved. Next, add 30.9 grams of 6.5% PVOH (as above) and mix well. Add another gram of Glyoxal 40L (Glyoxal 40L is Clariant's product name for glyoxal). Finally, 3.3 grams of Microlite 963 was added to the cup and the mixture was stirred for 5 seconds (Microlite is a trademark of W.R. Grace & Co. - Conn. and Microlite 963 is a 7.5% by weight suspension of vermiculite in water). Observe the rotational pattern of the particles, which indicates the alignment of the platelets in the plane of motion of the liquid. The glare occurs as a result of the platelets reflecting light from above.
[0130] The mixture was subjected to a "spin test" as defined above. After 10 minutes of spinning, ...
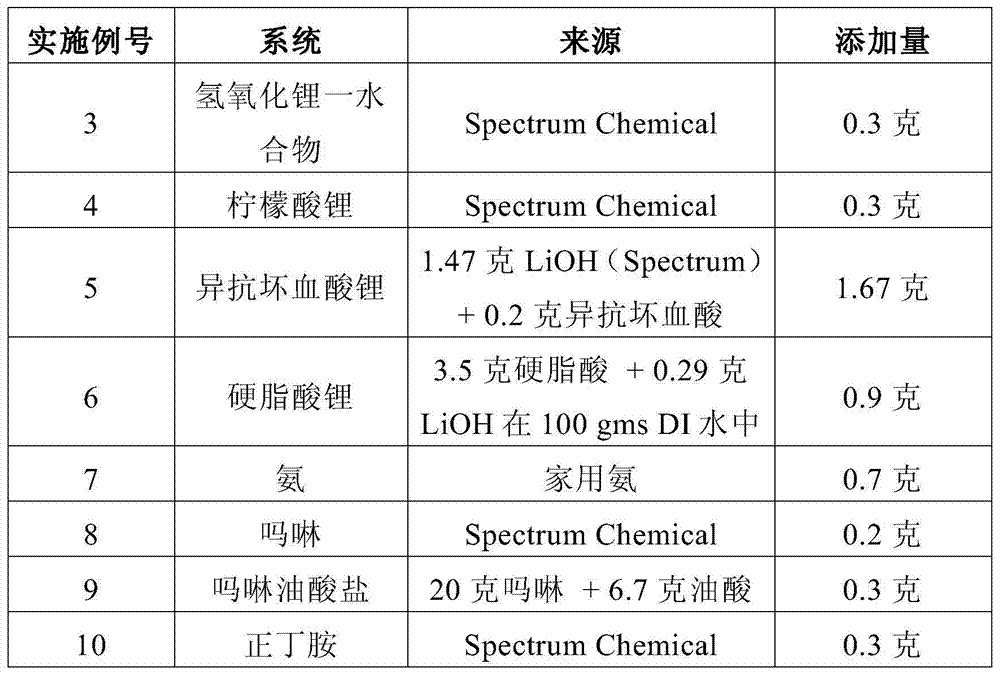
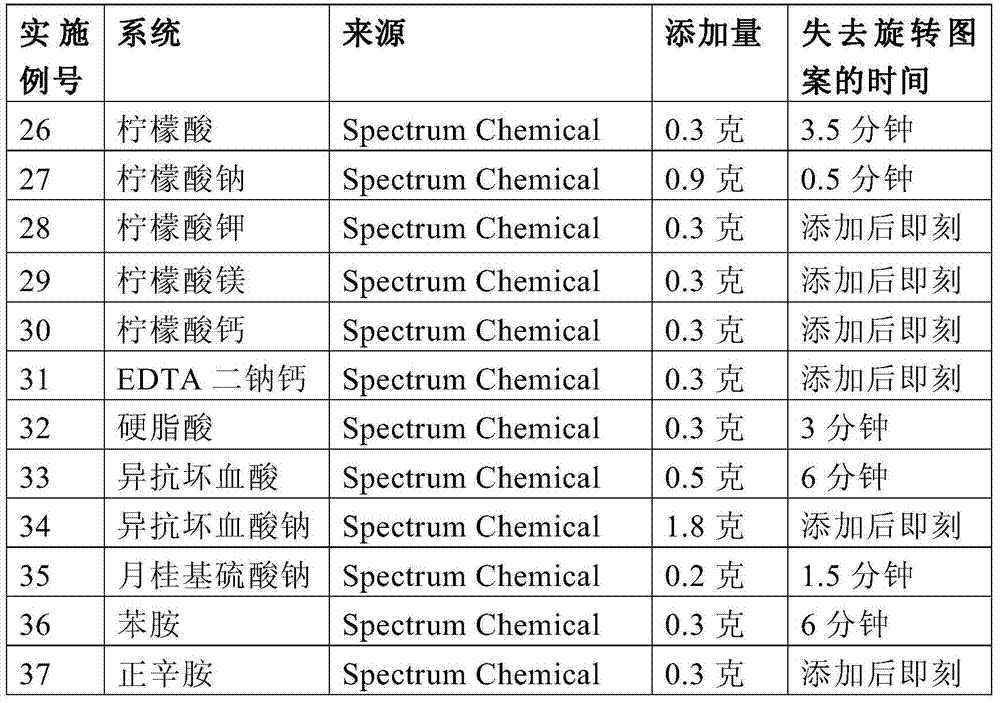
Embodiment 3-11
[0132] A mixture was prepared as in Example 2, substituting the processing aid listed in Table 1 for lithium hydroxide monohydrate. The mixture was subjected to a "spin test" as defined above. After 10 minutes, the swirling pattern and shiny light reflections of the Microlite963 particles were still there.
[0133] Table 1
[0134]
[0135] A series of experiments were performed to determine the effective lower limit for three of the stabilizers listed in Table 1 for the vermiculite clay platelets. The following examples can be applied to any stabilizer. The method is an iterative assay method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com