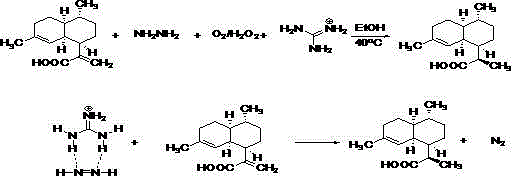
A kind of preparation method of dihydroartemisinic acid
A technology of dihydroartemisinic acid and artemisinic acid, applied in the direction of ozone oxidation to prepare carboxylic acid, organic chemistry, etc., can solve the problems of low reduction reaction temperature, high production energy consumption, long reaction time, etc., and achieve simple preparation process, The effect of reduced dosage and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Dissolve 10g of artemisinic acid in 50ml of absolute ethanol, then add 6.5ml of 50% hydrazine hydrate and 3ml of an aqueous solution containing 200mg of guanidine nitrate, adjust the reaction temperature to 45°C, and feed oxygen into the reaction solution at a rate of 60ml / min After 4 hours of reaction, HPLC showed that the reaction was complete; adding 100ml of water, extracted twice with 50ml of methyl tert-butyl ether, dried the organic phase with anhydrous sodium sulfate, filtered, concentrated, and the obtained residue was crude dihydroartemisinic acid 11.0g, the purity was 90% as shown by HPLC.
Embodiment 2
[0030] Dissolve 2.34g of artemisinic acid in 10ml of absolute ethanol, then add 2ml of 40% hydrazine hydrate and 1ml of an aqueous solution containing 40mg of guanidine sulfate, adjust the reaction temperature to 55°C, and slowly and uniformly drop 30% of hydrogen peroxide 6.0 into the reaction solution ml, the dropwise addition process lasted for 4 hours; after the dropwise addition was completed, HPLC showed that the reaction had been completed after 3 hours of heat preservation reaction; 50ml of water was added, extracted twice with 30ml of methyl tert-butyl ether, and the organic phase was dried with anhydrous sodium sulfate. After filtration and concentration, the obtained residue was 2.4 g of crude dihydroartemisinic acid, the purity of which was 92% as shown by HPLC.
Embodiment 3
[0032] Dissolve 10g of artemisinic acid in 50ml of absolute ethanol, then add 6.5ml of 80% hydrazine hydrate and 5ml of aqueous solution containing 200mg of guanidine hydrochloride, adjust the reaction temperature to 50°C, and feed oxygen into the reaction solution at a rate of 120ml / min After 4 hours of reaction, HPLC showed that the reaction was complete; adding 100ml of water, extracted twice with 50ml of methyl tert-butyl ether, dried the organic phase with anhydrous sodium sulfate, filtered, concentrated, and the obtained residue was crude dihydroartemisinic acid 10.5g, the purity was 91.8% by HPLC.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap