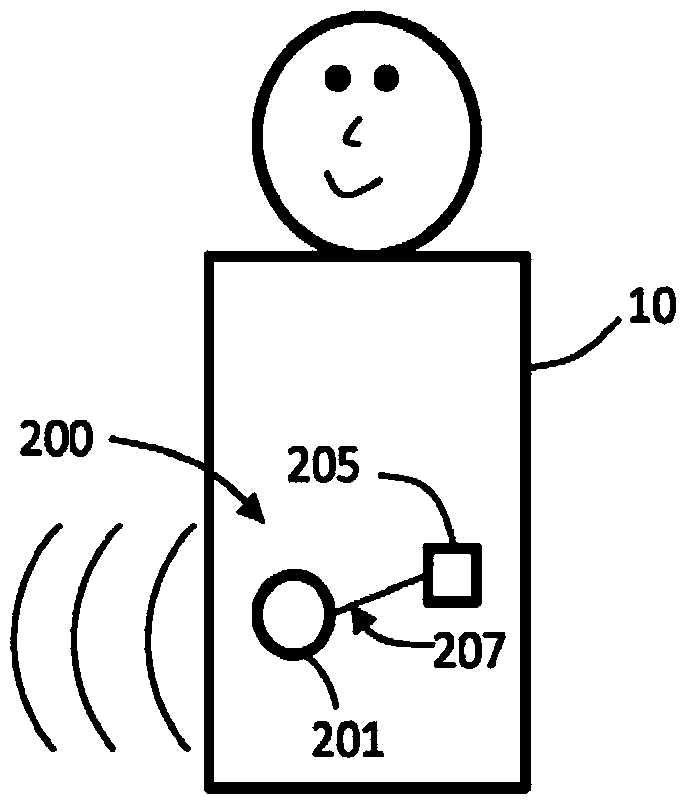
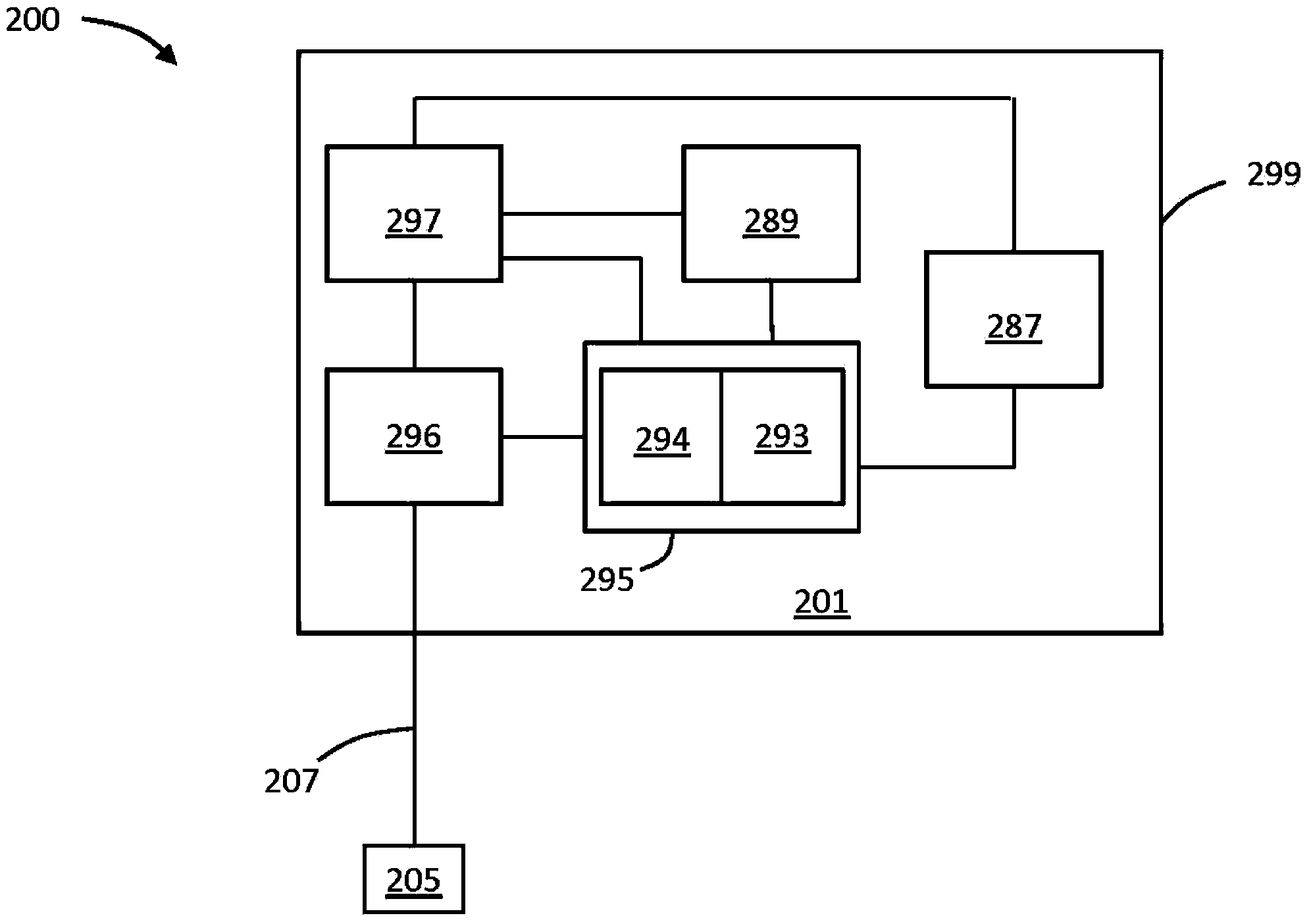
Monitoring fluid volume for patients with renal disease
A technology of liquid volume and liquid, which is applied in the field of liquid volume devices and can solve problems such as critical illness of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0100] The following prophetic examples serve to provide guidance on how to obtain and interpret data from an implantable sensor configured to measure tissue fluid in a patient in a use or device as described in the detailed description above quantity. It will be appreciated that the foreshadowing example presented here, consistent with the general principles of the case, is only one appropriate means for obtaining and interpreting the monitored data.
[0101] For this prophetic example, an implantable tissue impedance sensor such as the Medtronic A fluid status monitoring system is implanted in a patient to measure tissue impedance between the housing of the device and an electrode extending from the housing via a lead. Well-documented procedures for determining acceptable ranges for tissue impedance and fluid volume have been established. See, eg, (i) Siegenthalar, et al., Journal of Clinical Monitoring and Computing (2010):24:449-451 (Siegenthalar, et al. Journal of Clin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com