A kind of preparation method of hydroxyethyl starch-doxorubicin bond drug
A technology of hydroxyethyl starch and doxorubicin, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low drug loading and lack of intelligence in the release of doxorubicin and other issues to achieve enhanced drug effects, good biocompatibility, and easy modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
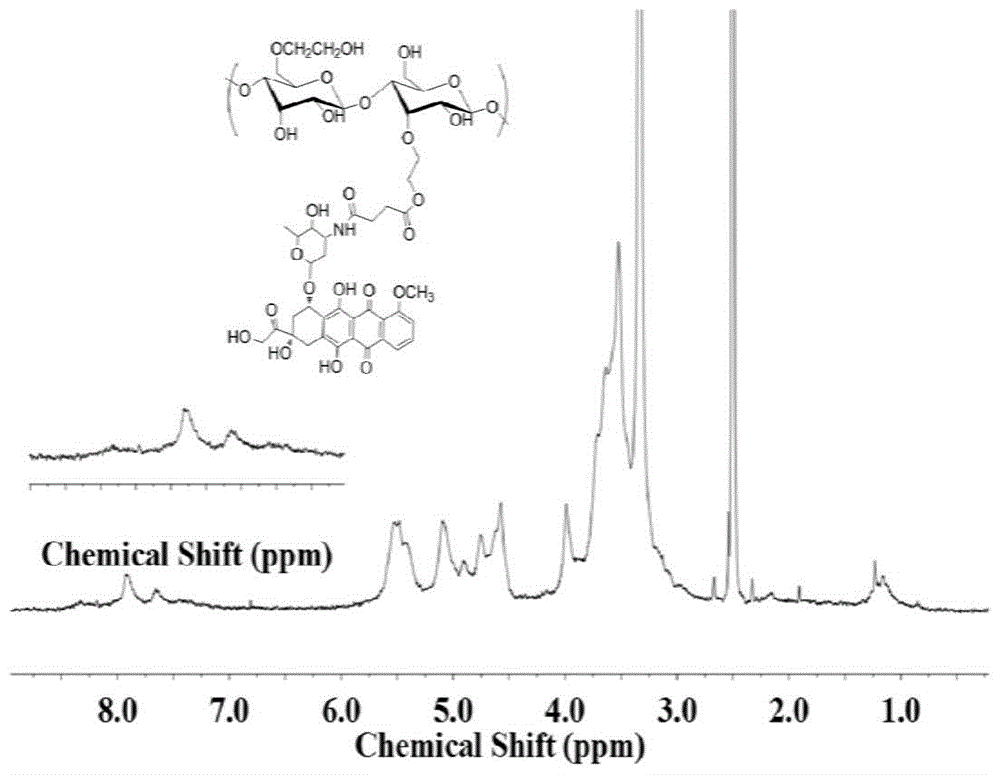
[0028] Put 0.4g of hydroxyethyl starch and 0.1ml of triethylamine in a dry reaction bottle, add 10ml of anhydrous dimethyl sulfoxide to dissolve, and dissolve 0.07g of p-nitrophenyl chloroformate in 5ml of anhydrous Dimethyl sulfoxide was added dropwise to the reaction flask at 25°C under stirring with a stirrer. After the dropwise addition, the reaction was continued for 24 hours. After the reaction was completed, the mixed solution was put into a dialysis bag for dialysis and freeze-dried to obtain formic acid. Hydroxyethyl starch modified with p-nitrophenyl ester. Carry out nuclear magnetic resonance analysis, infrared analysis to the hydroxyethyl starch modified by formic acid p-nitrophenyl ester obtained, by nuclear magnetic resonance analysis and infrared analysis, the hydroxyethyl starch modified by formic acid p-nitrophenyl ester prepared by the embodiment of the present invention has Formula (III) structure.
[0029] The obtained hydroxyethyl starch modified with p-n...
Embodiment 2
[0032] Put 0.4g of hydroxyethyl starch and 0.13ml of triethylamine in a dry reaction bottle, add 10ml of anhydrous dimethyl sulfoxide to dissolve, and dissolve 0.09g of p-nitrophenyl chloroformate in 5ml of anhydrous Dimethyl sulfoxide was added dropwise to the reaction flask at 25°C under stirring with a stirrer. After the dropwise addition, the reaction was continued for 24 hours. After the reaction was completed, the mixed solution was put into a dialysis bag for dialysis and freeze-dried to obtain formic acid. Hydroxyethyl starch modified with p-nitrophenyl ester. Carry out nuclear magnetic resonance analysis, infrared analysis to the hydroxyethyl starch modified by formic acid p-nitrophenyl ester obtained, by nuclear magnetic resonance analysis and infrared analysis, the hydroxyethyl starch modified by formic acid p-nitrophenyl ester prepared by the embodiment of the present invention has Formula (III) structure.
[0033] Put the obtained hydroxyethyl starch modified by ...
Embodiment 3
[0036] Put 0.4g of hydroxyethyl starch and 0.15ml of triethylamine in a dry reaction flask, add 10ml of anhydrous dimethyl sulfoxide to dissolve, and dissolve 0.12g of p-nitrophenyl chloroformate in 5ml of anhydrous Dimethyl sulfoxide was added dropwise to the reaction bottle under the condition of 25°C and stirred by a stirrer. After the dropwise addition, the reaction was continued for 24 hours. Nitrophenyl ester modified hydroxyethyl starch. Carry out nuclear magnetic resonance analysis, infrared analysis to the hydroxyethyl starch modified by formic acid p-nitrophenyl ester obtained, by nuclear magnetic resonance analysis and infrared analysis, the hydroxyethyl starch modified by formic acid p-nitrophenyl ester prepared by the embodiment of the present invention has Formula (I) structure.
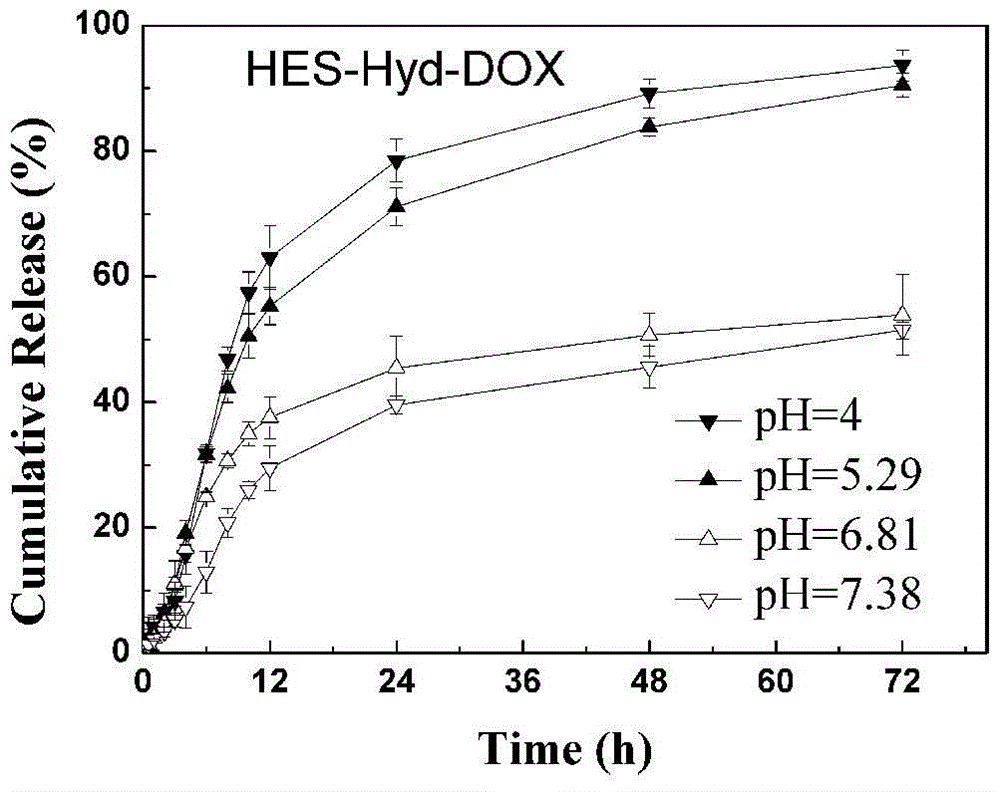
[0037] Put the obtained hydroxyethyl starch modified by p-nitrophenyl formate and 0.07ml of hydrazine hydrate into a reaction flask, add 10ml of anhydrous dimethyl sulfoxide to dissolv...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap