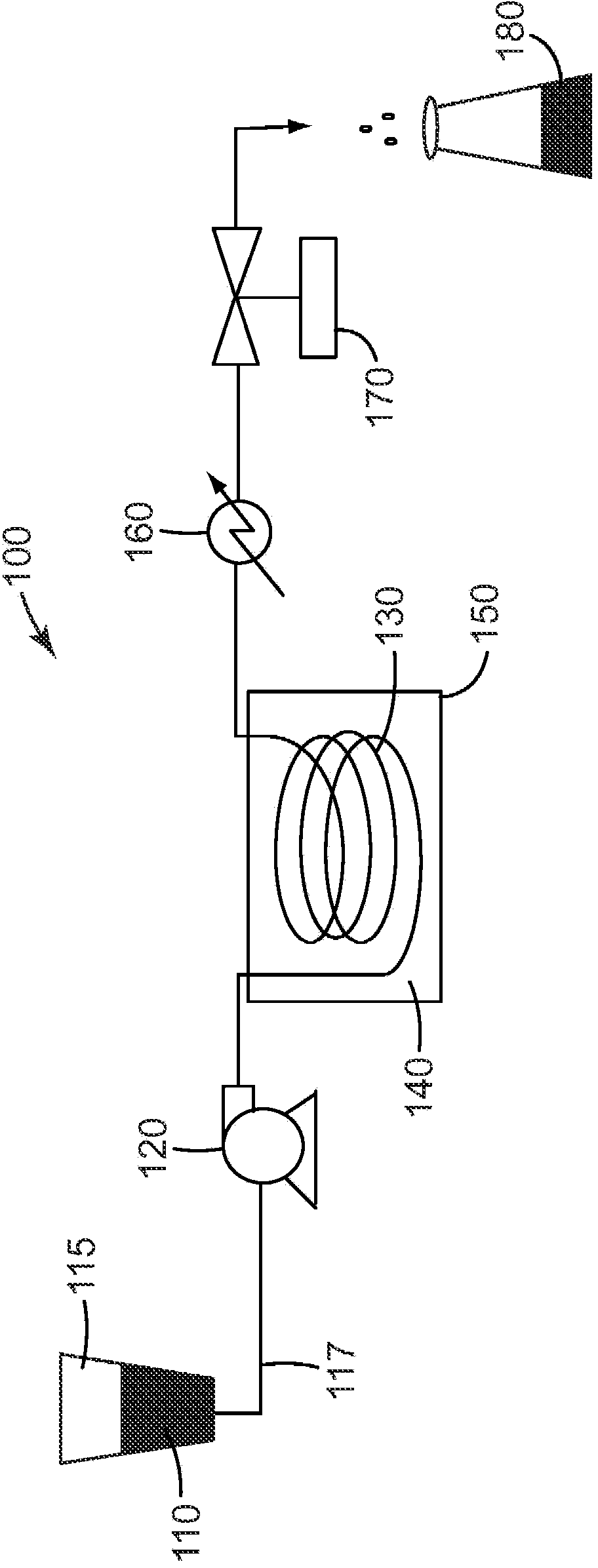
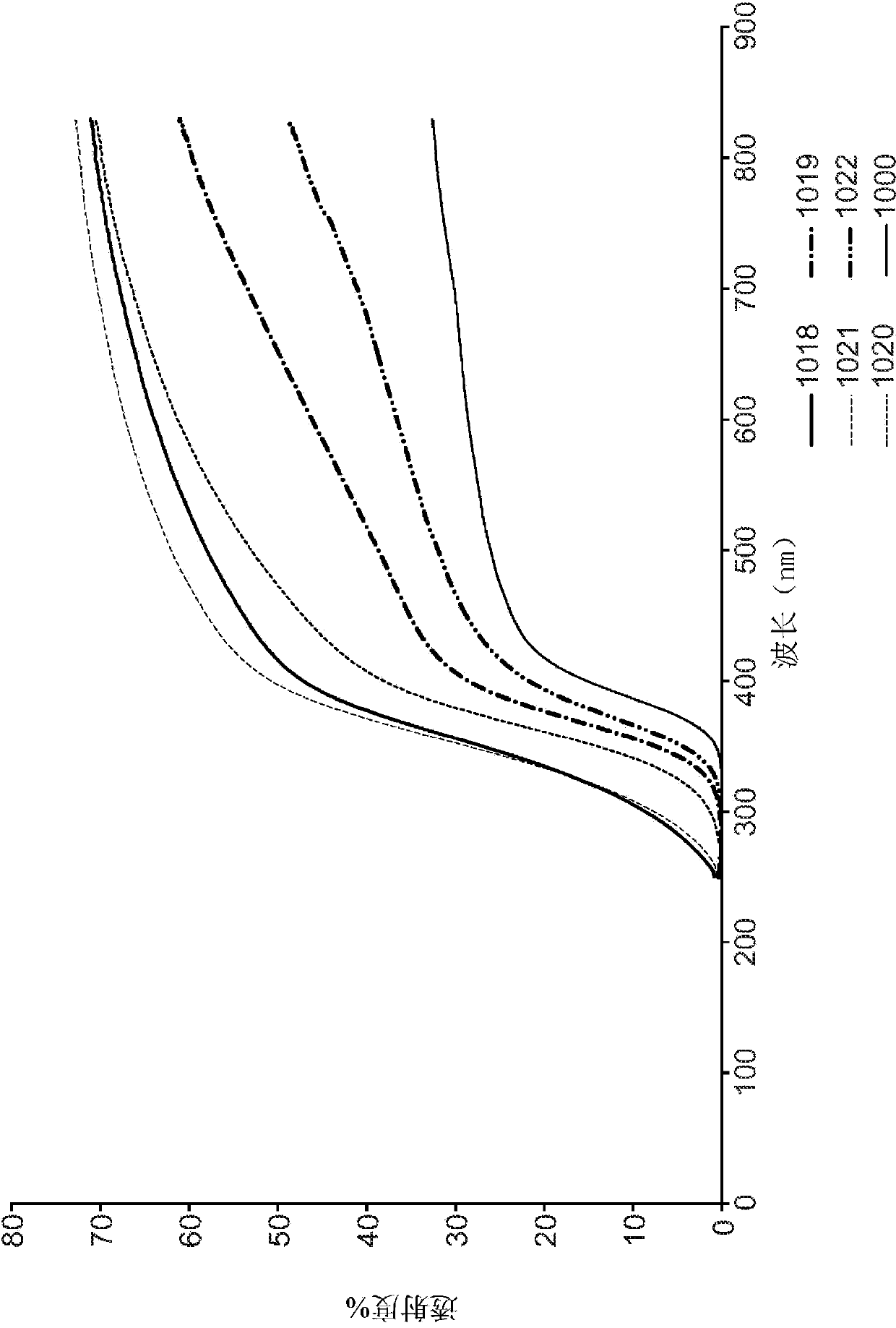
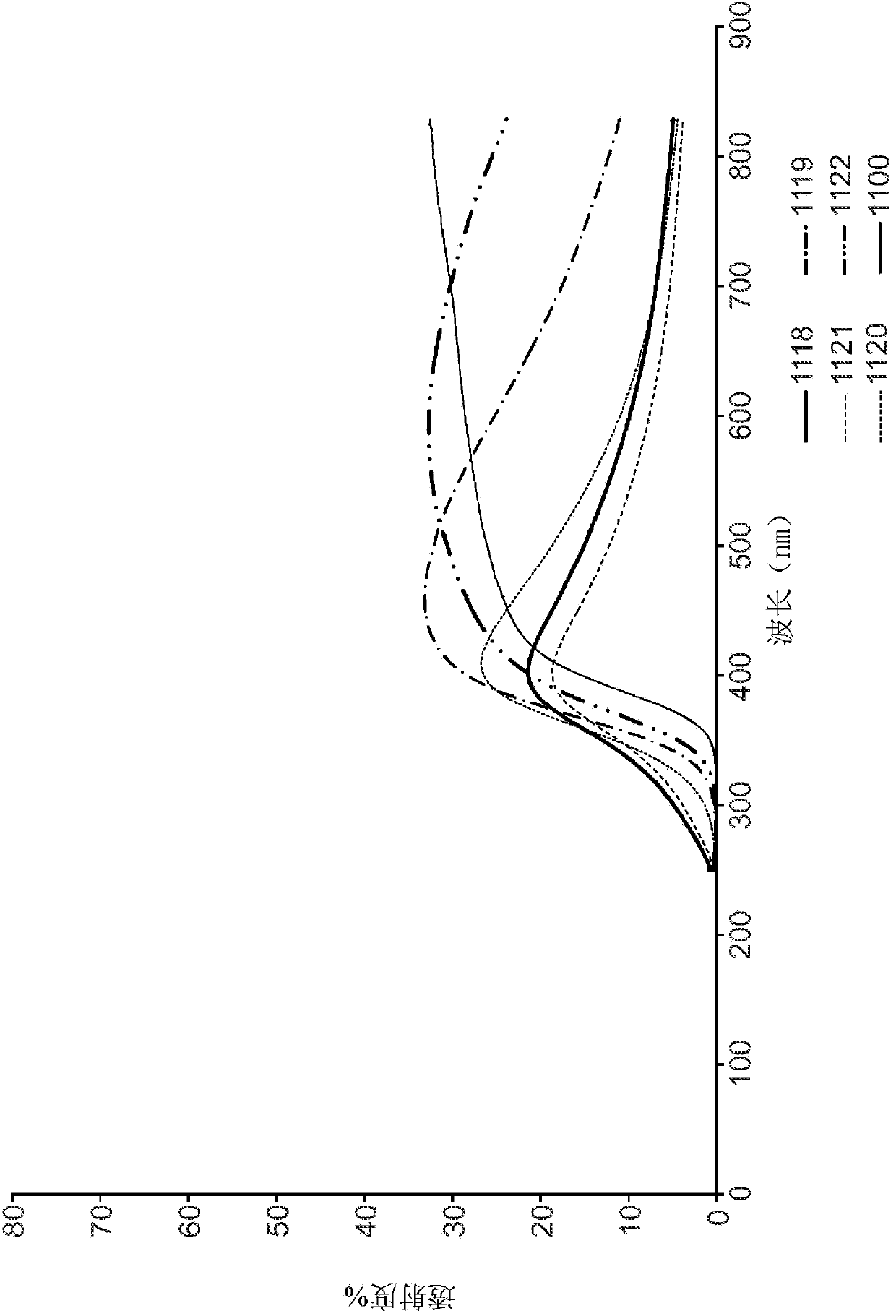
Aerogels, calcined and crystalline articles and methods of making the same
An airgel, crystallization technology with applications in chemical instruments and methods, ceramics, nanotechnology for materials and surface science, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] Depending on the method of preparation of the zirconia-based particles, said particles may comprise at least some organic material in addition to the inorganic oxide. For example, some organic material may be attached to the surface of the zirconia-based particles if the particles are prepared using a hydrothermal method. While not wishing to be bound by theory, it is believed that the organic material originates from carboxylate species (anions, acids, or both) contained in the feedstock, or is formed as a by-product of hydrolysis and condensation reactions (i.e., organic material is typically adsorbed on surface of zirconia-based particles). For example, in some embodiments, the zirconia-based particles comprise up to 15 (in some embodiments, up to 12, 10, 8, or even up to 6) weight percent organic material, based on the weight of the particles.
[0075] While any of a number of known methods may be used to provide the zirconia-based particles, it is preferred to emp...
example 1 and 2
[0454] To prepare Example 1, Sol S1 was diafiltered and concentrated as described above for Sol T1. The resulting sol was 55.4% by weight of ZrO 2 / Y 2 o 3 and 5.65% by weight of acetic acid. The sol (200 grams) was added to a 500 mL round bottom (RB) flask. Ethanol (60.6 grams), acrylic acid (11.5 grams) and HEMA (5.9 grams) were added to the flask. 2,2'-Azobis(2-methylbutyronitrile) ("VAZO67") (0.6 g) was added and the contents were stirred for 4 hours. then use N 2 The contents of the flask were purged with gas for 6 minutes. Samples (translucent and low viscosity) were added to cylindrical containers (29mm diameter). Each container is approximately 18 mL in volume and is sealed at each end (with a very small air gap between the top and the liquid). The samples were allowed to stand for about 1 hour and then placed into an oven to cure (50°C, 4 hours). This gave a clear translucent blue gel. The gel was removed from the container and placed into a 473 mL jar. Fil...
example 3
[0464] A 277 gram sample of Sol T1 (prepared as above and diafiltered and concentrated, 29.5 wt% oxide and 3.2 wt% acetic acid) was added to a 500 mL round bottom (RB) flask. Water (127 g) was removed via rotary evaporation to obtain a slightly dry viscous material. Ethanol (45.5 grams), acrylic acid (8.6 grams) and 2-hydroxyethyl methacrylate (HEMA) (4.4 grams) were added to the flask. The contents were stirred for about 4 hours to obtain a translucent fluid sol. 2,2'-Azobis(2-methylbutyronitrile) ("VAZO67") (0.45 g) was added and the contents were stirred for 5 minutes. then use N 2 The contents of the flask were purged with gas for 4 minutes. Samples (translucent and low viscosity) were added to cylindrical containers (29mm diameter). Each container is approximately 18 mL in volume and is sealed at each end (with a very small air gap between the top and the liquid). The samples were allowed to stand for about 1 hour and then placed into an oven to cure (50°C, 4 hours)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com