Telechelic quadruple-hydrogen bond unit compound and synthesis method thereof
A technology of quadruple hydrogen bond and synthesis method, applied in the direction of organic chemistry, can solve problems such as unsatisfactory mechanical properties, and achieve the effects of increasing the total yield, saving resources, and high mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
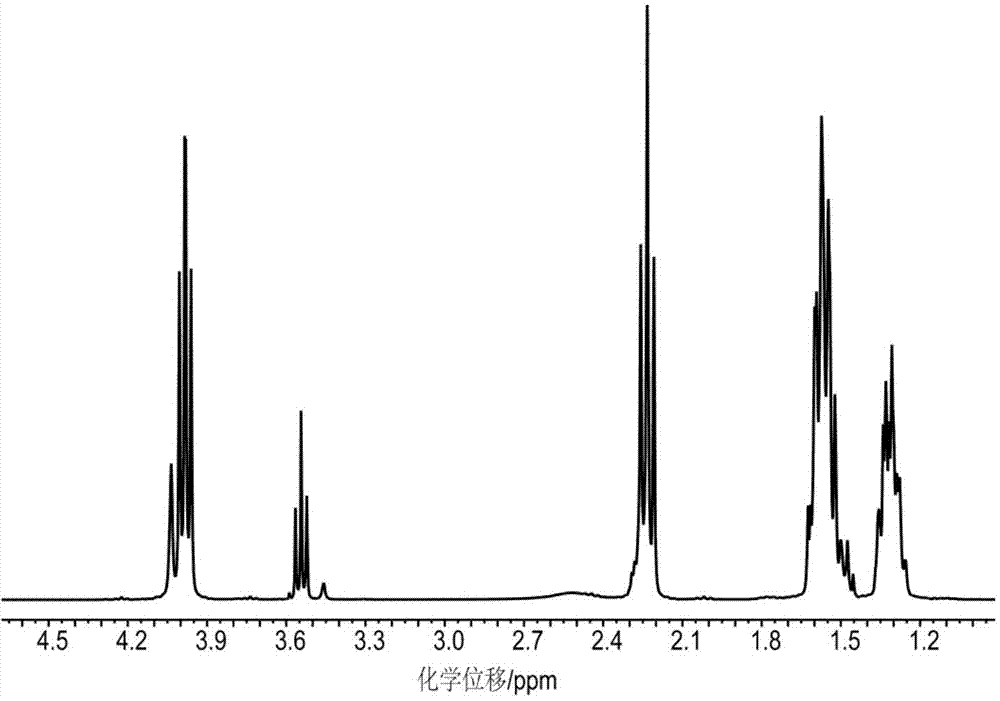
[0035] Step 1. Synthesis of intermediate linker pentaerythritol-polycaprolactone (PER-PCL) (compound A):
[0036] First, 1.36 g of pentaerythritol, 22.80 g of caprolactone and a catalytic amount of stannous octoate were added to a 100 mL round bottom flask. The oxygen and moisture in the system are eliminated through 3 to 5 consecutive vacuum pumping and argon filling cycles. Next, under the protection of nitrogen, the temperature was maintained at 140° C., the reaction was stirred for 10 h, and the heating was stopped. Continue stirring with nitrogen until the temperature of the reactant drops below 60°C and stop. Finally, under the protection of nitrogen, the reacted polycaprolactone is dissolved in chloroform after cooling, and then precipitated with deionized water for 2 to 3 times. After suction filtration, it can be dried at 40°C for 12 hours under vacuum. The product is a white solid. , the degree of polymerization n is 5, and the yield is 98%.
[0037] The product w...
Embodiment 2~5
[0045] The synthesis of compound A was synthesized with reference to step 1 of Example 1, only changing the molar ratio of caprolactone: pentaerythritol to 40:1, 60:1, 80:1 and 100:1, the degree of polymerization n of the obtained compound A were respectively for 10, 15, 20 and 25. The synthesis of compound B and compound C is the same as in Example 1.
Embodiment 6
[0047] Step 1, the synthesis of compound A was synthesized with reference to Example 1.
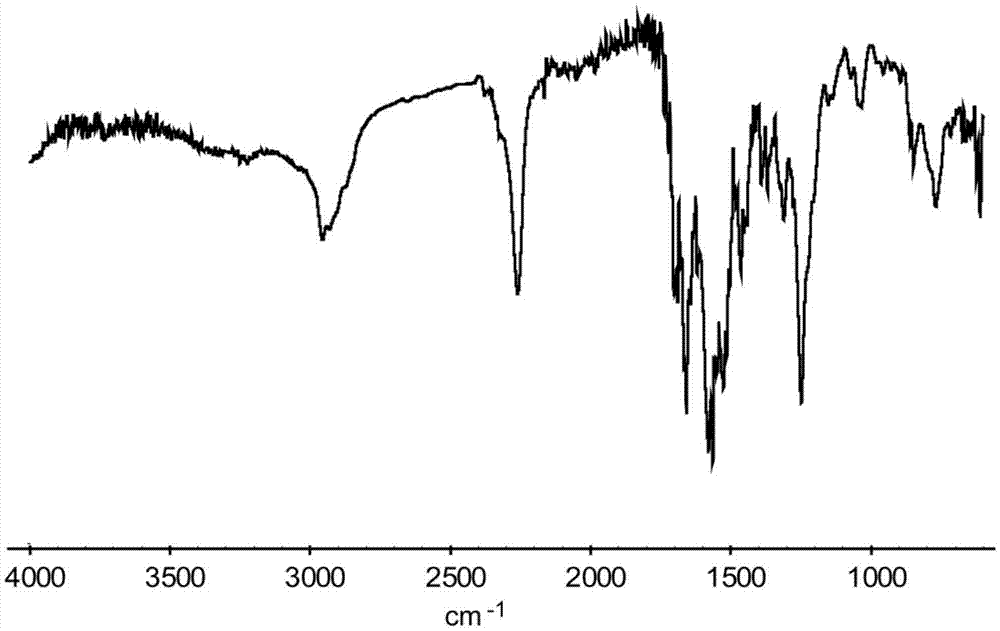
[0048] Step 2, synthesis of quadruple hydrogen bond unit (compound B):
[0049] First, add 1.25 g of MIC and 17.50 g of diphenylmethane diisocyanate (MDI) in a 100 mL round bottom flask. The oxygen and moisture in the system are eliminated through 3 to 5 consecutive vacuum pumping and argon filling cycles. Next, under the protection of argon, the temperature was maintained at 110° C., the reaction was stirred for 12 h, and the heating was stopped. Continue stirring with argon until the temperature of the reactant drops to room temperature and stops. Finally, the reacted reaction mixture was dissolved with a little chloroform, and then precipitated with n-hexane for 2 to 3 times, and then dried under vacuum at 50° C. for 12 hours after suction filtration.
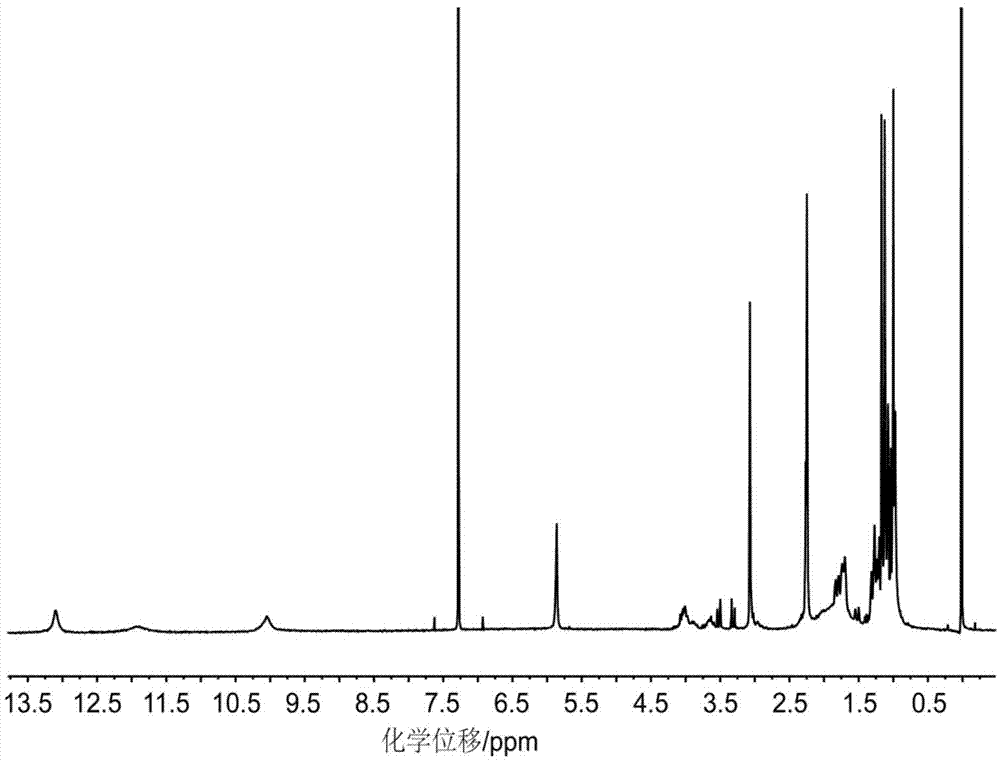
[0050] Step 3, synthesis of target compound (compound C):
[0051] First, add the above 2.35g compound A and 5.64g compound B, 10m...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap