Preparation and application of anti-Staphylococcus aureus eLtaS protein monoclonal neutralizing antibody E4-2
A monoclonal antibody, Staphylococcus technology, applied in the direction of anti-bacterial immunoglobulin, antibody, anti-bacterial drugs, etc., to achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Construction of anti-eLtaS monoclonal antibody hybridoma cell line
[0036] Materials: Freund's complete adjuvant and incomplete adjuvant, TMB is a product of Sigma, 20% fetal bovine serum is a product of Beijing Yuanheng Shengma Biotechnology Research Institute, serum-free RPMI1640 is a product of Gibco, SP2 / 0 cells are from ATCC Imported and kept in our laboratory, Balb / c mice and Kunming mice were purchased from the Experimental Animal Center of Academy of Military Medical Sciences. The rest of the reagents were purchased from the market.
[0037] Method result:
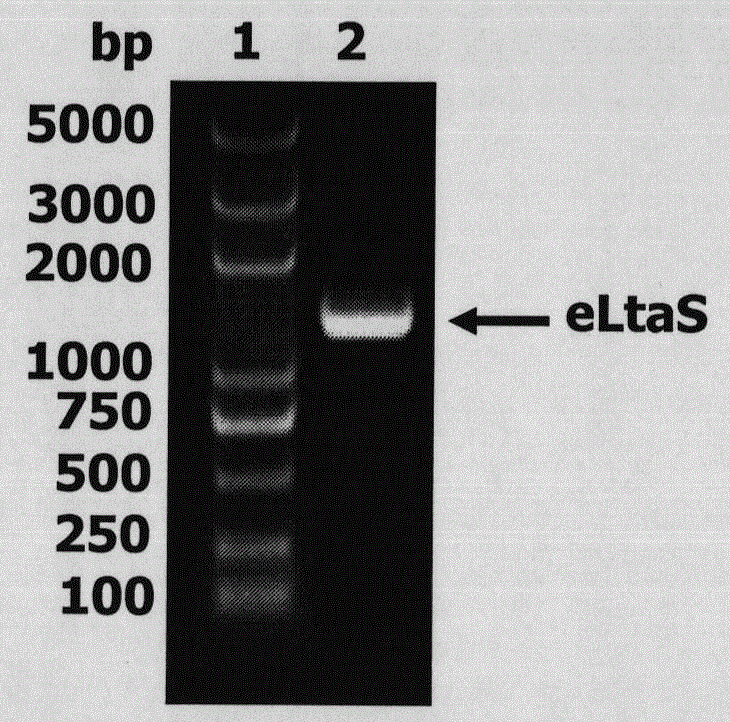
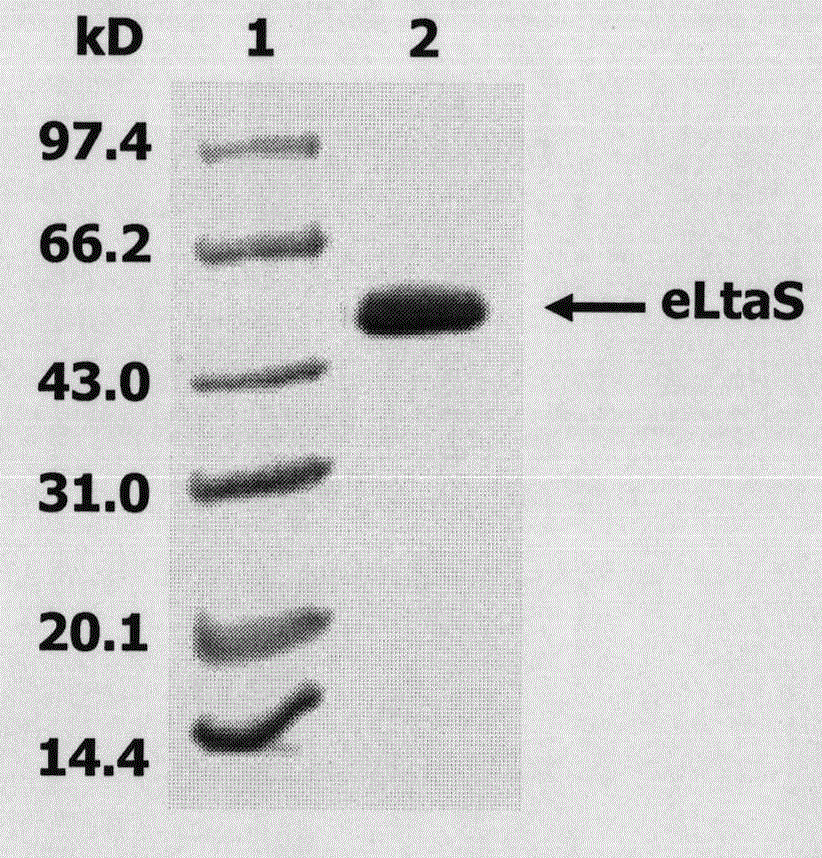
[0038] 1. Expression and purification of eLtaS protein. The eLtaS gene in the genome of Staphylococcus aureus 8325-4 was fished out by using eLtaS coding region-specific primers (the electrophoresis detection of it was as follows: figure 1 shown), and cloned into the pET-28a vector to induce, express, and purify eLtaS protein (the SDS-PAGE results are shown in figure 2 ) shown.
[0039] 2...
Embodiment 2
[0044] Example 2 Screening and Identification of Anti-eLtaS Neutral Monoclonal Antibody Hybridoma Cell Lines
[0045] Material: Protein G Sepharose CL4B column: product of GE healthcare company; Ibid.
[0046] Method result:
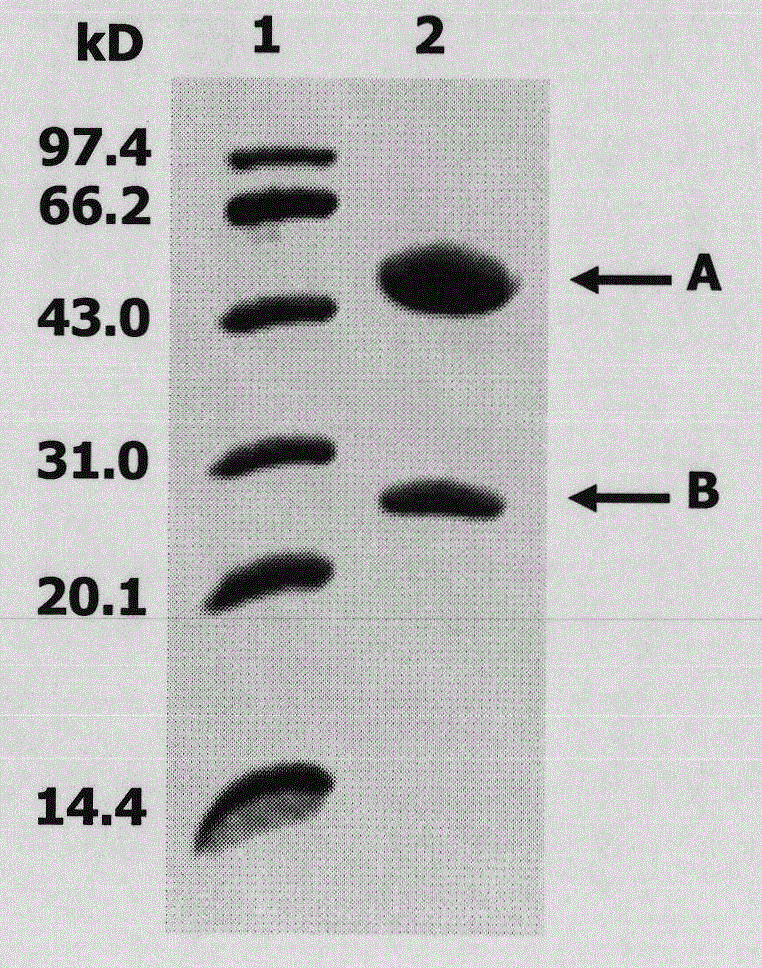
[0047] 1. Purification of eLtaS monoclonal antibody. Add 1 ml of pH8.0, 0.1moL / L phosphate buffer to 2 ml of mouse ascites and adjust the pH to 9 with pH9.0, 1moL / L TRIS-HCL. Add the mouse ascites to the Protein G Sepharose CL4B column protein column that has been equilibrated with 0.1moL / L phosphate buffer solution pH8.0, and wash the column with the above buffer solution until no foreign protein can be detected in the effluent. Elute with citric acid buffer of pH 3.0, collect the effluent, and immediately neutralize with 1moL / L TRIS-HCL pH 8.5 buffer, and dialyze with pH 7.2, 0.01M PBS for 72h. Sampling for SDS-PAGE detection ( image 3 ).
[0048] 2. Screening of neutralizing antibodies. Use brain heart infusion medium to culture Staphylococcus ...
Embodiment 3
[0053] Example 3 The protective experiment of monoclonal antibody E4-2 to CD1 mice
[0054] Materials: Same as Examples 1 and 2 above.
[0055] Method result:
[0056] 1. The acute peritonitis model was used to detect the effect of anti-eLtaS monoclonal antibody E4-2 against Staphylococcus aureus infection. 6-week-old female CD1 mice were treated with intraperitoneal injection of PBS, control IgG (100 μg) and E4-2 (100 μg); the single clone of Staphylococcus aureus 8325-4 was picked and cultured in brain heart infusion medium , 37°C, 200rpm for 12hr. After the bacteria were collected, the bacteria were washed once with PBS, and the concentration of the bacteria was adjusted to OD600=1.5, and 500 μl was injected intraperitoneally into the mice, and the survival number of mice at different time points was observed ( Figure 7 ).
[0057] 2. The pneumonia model was used to test the anti-eLtaS monoclonal antibody E4-2 against Staphylococcus aureus infection. 6-week-old female...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com