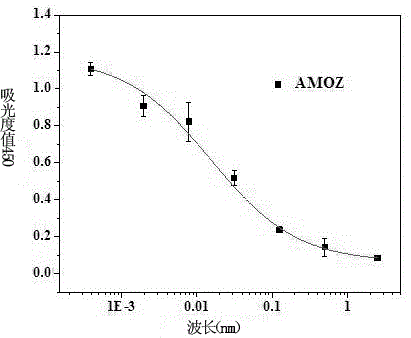
A rapid immunoassay for the direct detection of furaltadone metabolite amoz
An immunodetection method and furatanone technology are applied in immunoglobulins, chemical instruments and methods, biological tests, etc., to achieve the effects of strong professionalism, high cost and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of hapten HI:
[0033] Take 604mg (3mmol) of AMOZ and 266.5mg (3.6mmol) of glyoxylic acid and dissolve them in 2mL of absolute ethanol respectively, add the ethanol solution of glyoxylic acid to the ethanol solution of AMOZ dropwise during the stirring process, and stir at room temperature overnight. After the reaction was completed, vacuum filtration was performed, and the filter cake was washed twice with 2 mL of ethanol and diethyl ether successively, and the precipitate was collected to obtain 540.2 mg of the target product HI as a white solid, with a yield of 70%. ESI-MSanalysis (positive) m / z258.7[M+H]+; 1HNMR(600MHz,d5-Pyridine,TMS):δ7.56(s,1H),4.99-4.97(m,1H),4.16(t ,J=8.8Hz,1H),3.79(dd,J1=6.7,J2=8.8,1H),3.68-3.64(m,4H),2.66(dd,J1=6.1,J2=13.5,1H),2.62( dd, J1=5.5, J2=13.4, 1H), 2.51-2.46(m, 4H).
Embodiment 2
[0034] Example 2 Preparation method of hapten HII
[0035] Take 604mg (3mmol) of AMOZ and 353mg (3.6mmol) of maleic anhydride and dissolve them in 2mL of absolute ethanol respectively, add the ethanol solution of maleic anhydride to the ethanol solution of AMOZ dropwise during the stirring process, and stir at room temperature to react overnight. After the reaction was completed, vacuum filtration was performed, and the filter cake was washed twice with 2 mL of ethanol and ether, and the precipitate was collected to obtain 566 mg of the target substance HII as a white solid with a yield of 63%. ESI-MS analysis (negative) m / z298.6[M-H]-; 1HNMR(500MHz,d6-DMSO,TMS):δ6.32(d,J=2.9Hz,2H),4.86–4.78(m,1H), 3.74(t, J=8.1Hz, 2H), 3.58(s, J=4.6Hz, 4H), 3.43(t, J=7.7Hz, 1H).
Embodiment 3
[0036] Example 3 Immunogen / coating preparation
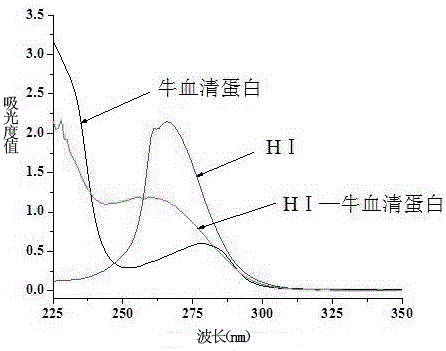
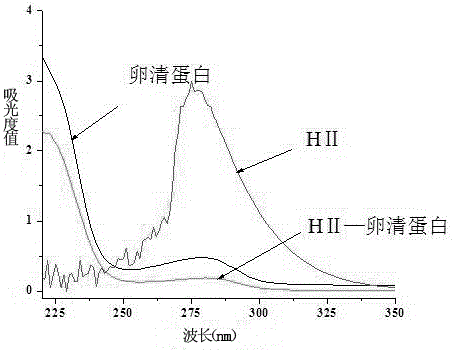
[0037] The difference between the preparation of the immunogen and the coating source lies in the structure of the hapten and the type of carrier protein. The immunogen uses the hapten HI, and the carrier protein uses bovine serum albumin (BSA); the coating source uses the hapten HII. The carrier protein is ovalbumin (OVA).
[0038] Methods of Immunogen Preparation. Active ester method: Take 10 mg (0.04 mmol) of hapten HⅠ, dissolve in 500 μL dimethylformamide (DMF), stir and add 20.6 mg (0.1 mmol) 1,3-dicyclohexylcarbodiimide (DCC) and 11.5 mg (0.1 mmol) of N-hydroxysuccinimide (NHS), react overnight at 4°C with magnetic stirring, centrifuge and take the supernatant as liquid A. Weigh 20 mg of carrier protein BSA and dissolve in 2 mL of PBS solution (0.1 mol / L, pH 7.4), stir and dissolve to prepare solution B. Under magnetic stirring, absorb 384 μL of solution A and add it dropwise to solution B, and stir and react at 4°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com