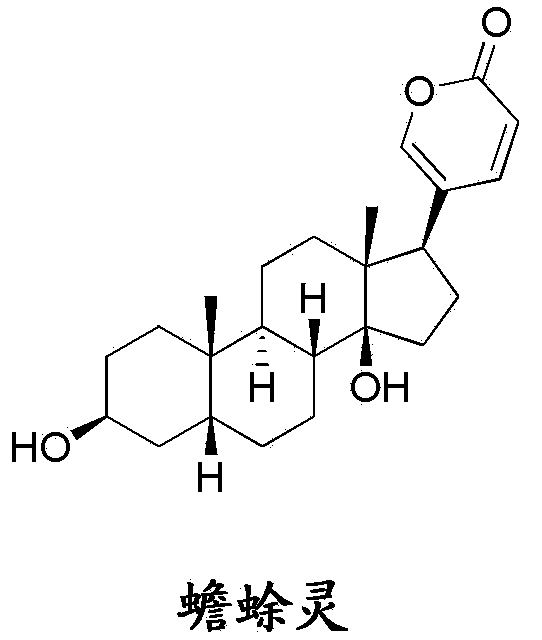
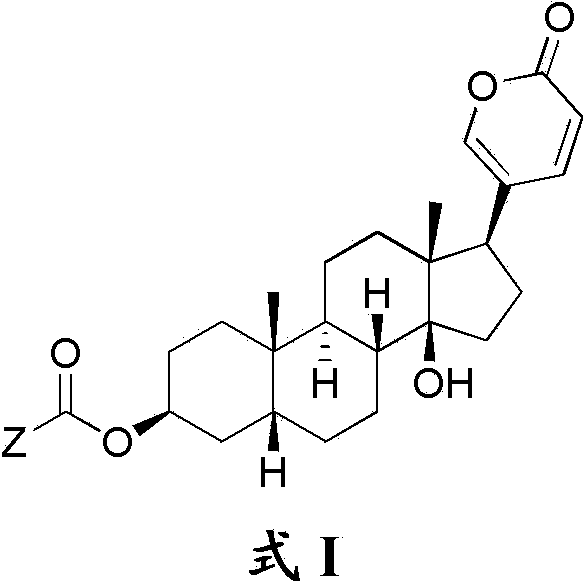
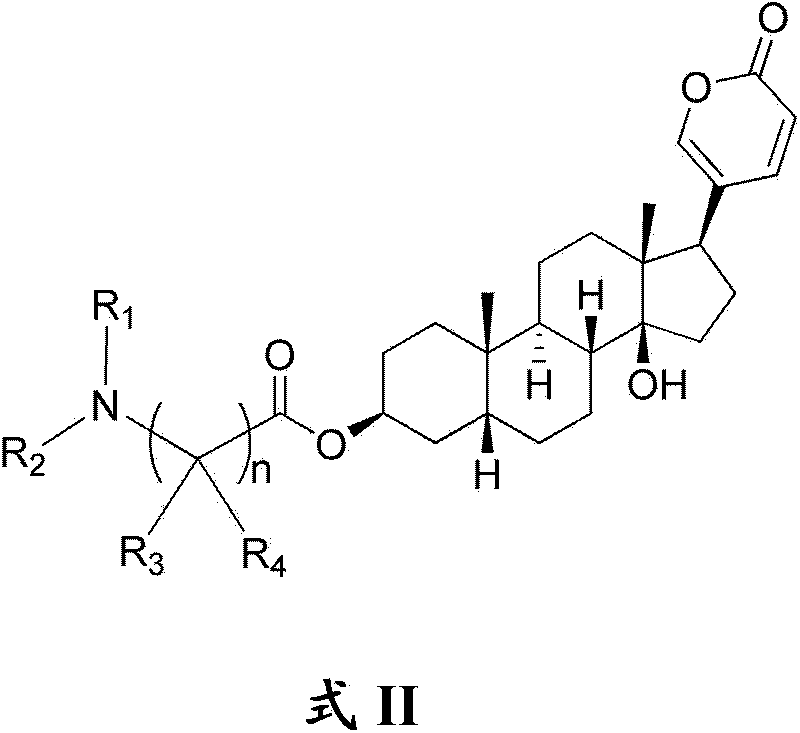
Bufalin derivatives, pharmaceutical compositions and methods thereof
A compound and pharmaceutical technology, applied in the field of its pharmaceutical composition and bufalin derivatives, can solve the problems of poor solubility, narrow therapeutic index, limitation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0270] The following examples serve to more fully describe the manner of using the invention. These examples are provided for illustrative purposes and should not be used to limit the true scope of the invention.
[0271] In practicing the steps of the methods described herein, it will of course be understood that references to particular buffers, media, reagents, cells, culture conditions, etc., are not intended to be limiting, but are to be understood to include those that would be recognized by one of ordinary skill in the art when such references are made. All relevant material that is of interest or value in the particular context of the discussion. For example, it is often possible to substitute one buffer system or medium for another and still obtain similar, if not identical, results. Those skilled in the art have sufficient knowledge of such systems and methods to be able (without undue experimentation) to make such substitutions that best serve their purposes in usi...
Embodiment I
[0272] Example I: (R)-2-alanine (3S, 5R, 8R, 9S, 10S, 13R, 14S, 17R)-14-hydroxyl-10,13-dimethyl-17-(2-oxo Preparation of -2H-pyran-5-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3-yl ester
[0273]
[0274] (R)-2-aminopropionic acid (3S, 5R, 8R, 9S, 10S, 13R, 14S, 17R)-14-hydroxy-10,13-dimethyl-17-(2-oxo-2H-pyridine Fyran-5-yl)hexadecahydro-1H-cyclopentadien[a]phenanthrene-3-yl ester
[0275]
[0276] To Boc-amino acid (11.3mg, 0.06mmol, 1.2eq), HOBT (9.7mg, 0.072mmol, 1.44eq), EDC (13.8mg, 0.072mmol, 1.44eq) and DMAP (16.8mg, 0.15mmol, 3eq) in CH 2 Cl 2 Bufalin (20 mg, 0.05 mmol) was added to the solution in . The mixture was stirred at 37 °C for 16 h, then purified by preparative TLC (PE / EA=1:1) to give (R)-2-((tert-butoxycarbonyl)amino)propanoic acid (3S, 5R, 8R, 9S, 10S, 13R, 14S, 17R)-14-hydroxy-10,13-dimethyl-17-(2-oxo-2H-pyran-5-yl)hexadecahydro-1H-cyclopentadiene An[a]phenanthrene-3-yl ester (23 mg, 79.8%).
[0277]
[0278]At 0°C, to (R)-2-((tert-buto...
Embodiment II
[0279] Example II: (2-(pyrrolidin-1-yl)ethyl)carbonate (3S,5R,8R,9S,10S,13R,14S,17R)-14-hydroxy-10,13-dimethyl-17 Preparation of -(2-oxo-2H-pyran-5-yl)hexadecahydro-1H-cyclopentadien[a]phenanthrene-3-yl ester
[0280]
[0281] (2-(Pyrrolidin-1-yl)ethyl)carbonic acid (3S, 5R, 8R, 9S, 10S, 13R, 14S, 17R)-14-hydroxy-10,13-dimethyl-17-(2- Oxo-2H-pyran-5-yl)hexadecahydro-1H-cyclopenta[a]phenanthrene-3-yl ester
[0282]
[0283] To 1 (60mg, 0.15mmol) and DMAP (16.8mg, 0.15mmol) in CH 2 Cl 2 To a solution in (10 mL) was added DIEA (77.4 mg, 0.6 mmol) and 4-nitrophenyl carbonochloridate (60.6 mg, 0.3 mmol). The mixture was stirred at 37 °C for 16 h, then purified by preparative TLC (PE / EA=1:1) to give 4-nitrophenylcarbonic acid (3S, 5R, 8R, 9S, 10S, 13R, 14S , 17R)-14-hydroxyl-10,13-dimethyl-17-(2-oxo-2H-pyran-5-yl)hexadecahydro-1H-cyclopentadiene[a]phenanthrene-3 -yl ester (72mg, 87.1%).
[0284]
[0285] To 4-nitrophenylcarbonic acid (3S, 5R, 8R, 9S, 10S, 13R, 14S, 17...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com