Chlamydia antigen compositions and uses thereof
A composition and technology of Chlamydia are applied in the field of peptides and polypeptides against Chlamydia infection, and can solve problems such as inability to develop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
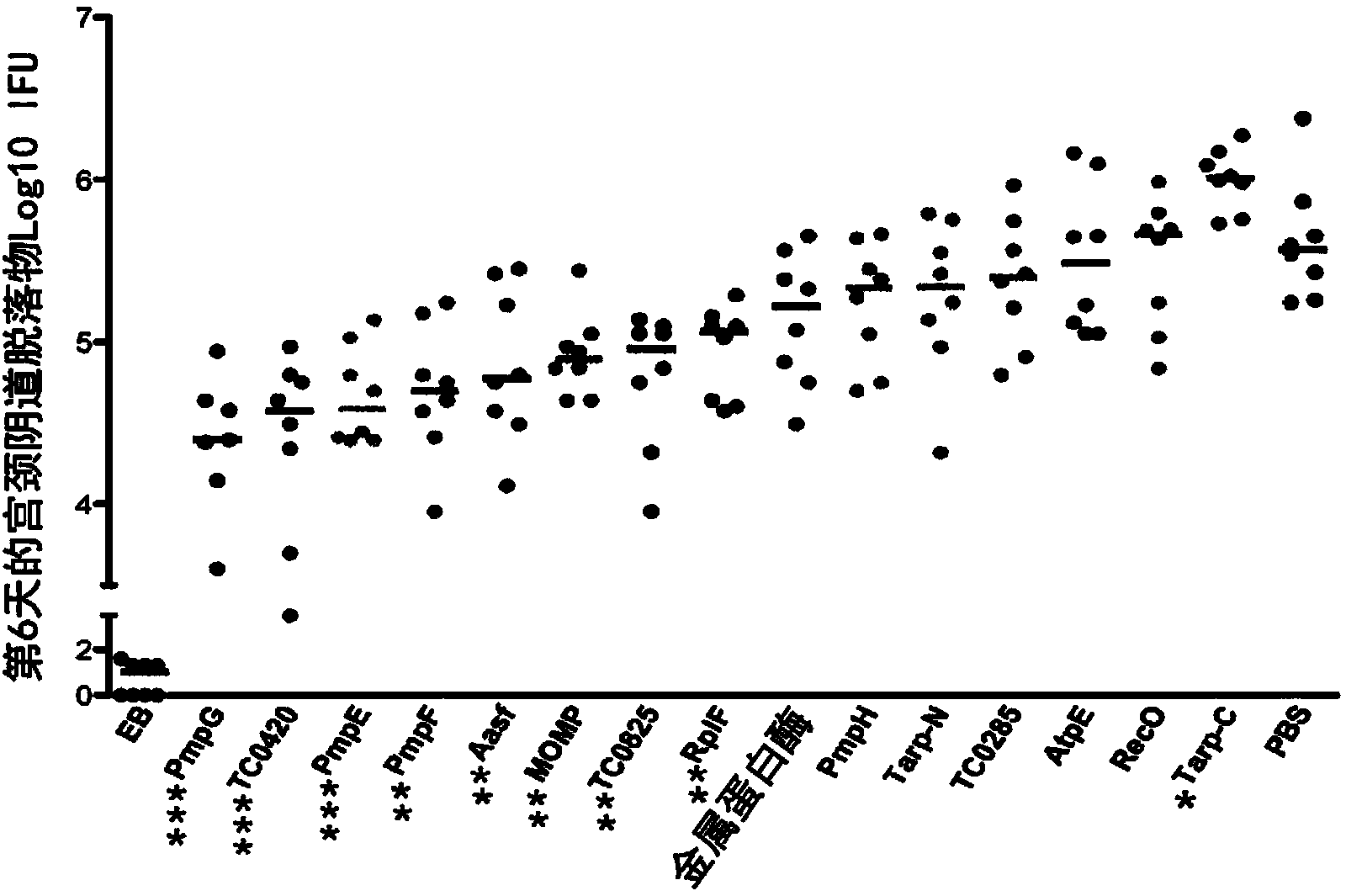
[0118] Materials and Methods
[0119] Chlamydia
[0120] The Chlamydia muris mouse pneumonia (MoPn) strain Nigg was grown in Hela229 in Eagle's minimal essential medium (Invitrogen) supplemented with 10% FCS. Primary bodies (EBs) were purified by discontinuous density gradient centrifugation as previously described (Caldwell, H.D., J. Kromhout and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun31 :1161-1176). Purified EBs were aliquoted and stored at -80°C in sucrose-phosphate-glutamate buffer and thawed before use. The infectivity and the number of inclusion-forming units (IFU) of purified EB were determined by using anti-EB mouse polyclonal antibody, followed by biotin-labeled anti-mouse IgG (Jackson ImmunoResearch Laboratories) and diaminobenzidine (DAB ) substrate (Vector Laboratories) immunostaining to determine (Yang, X., K.T.HayGlass and R.C.Brunham.1996.Genetically determined di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com