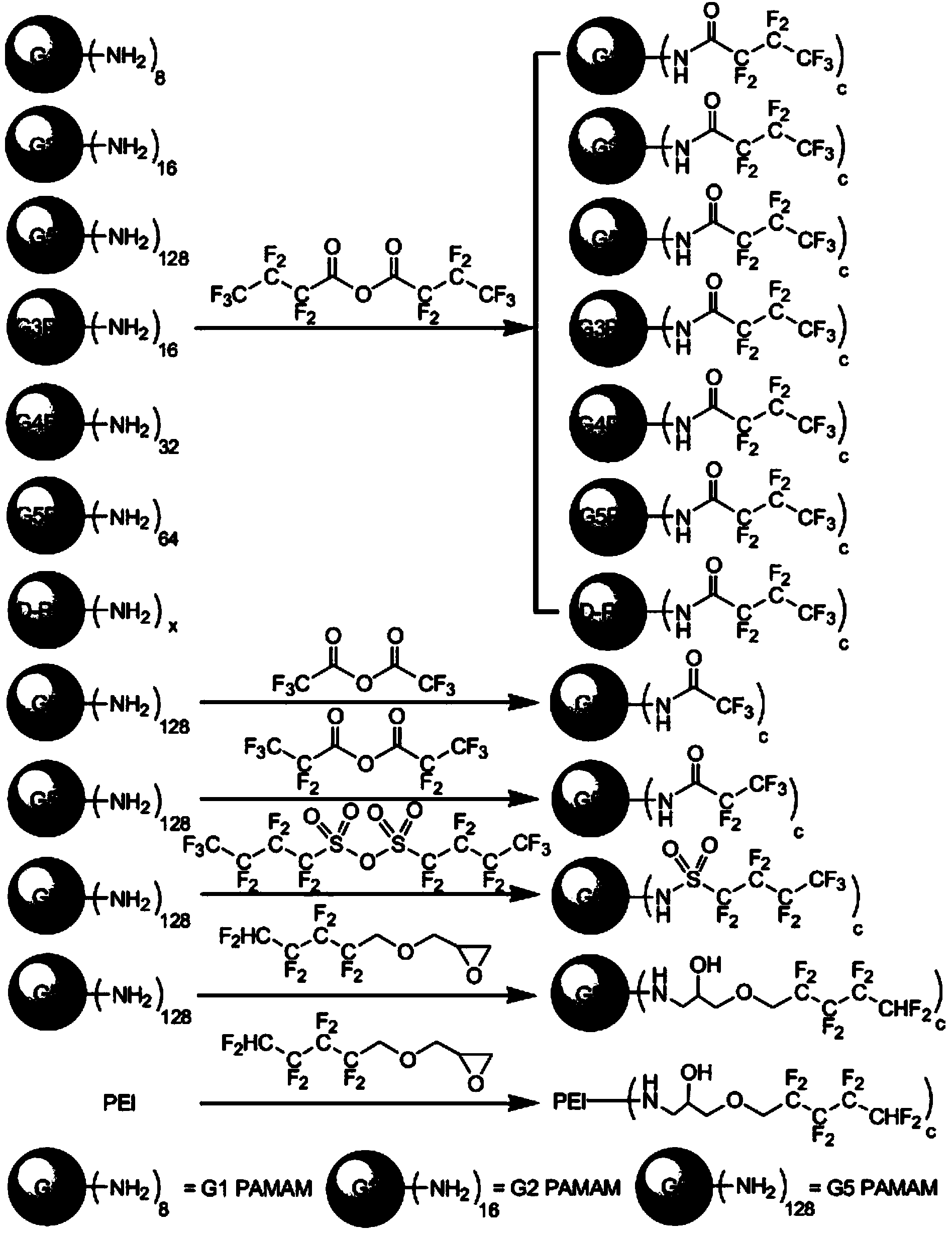
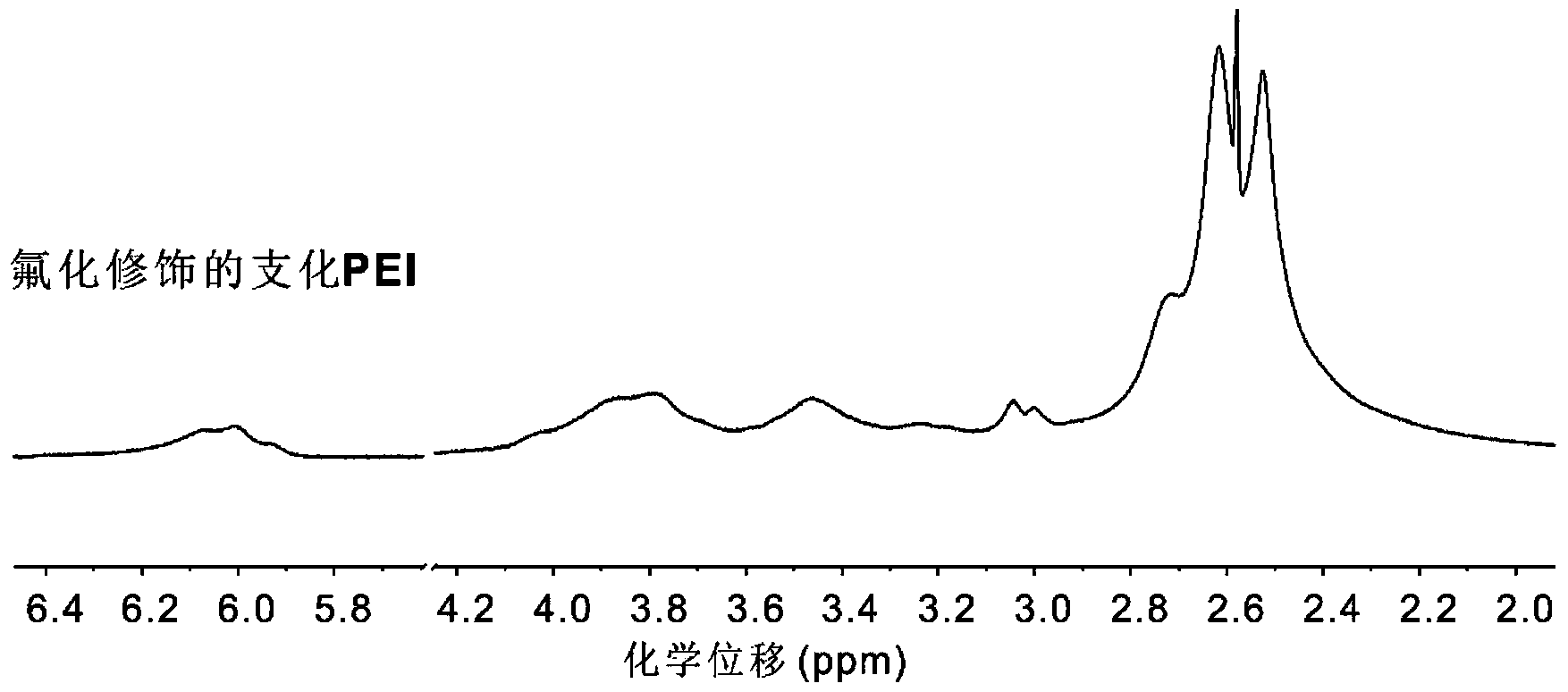
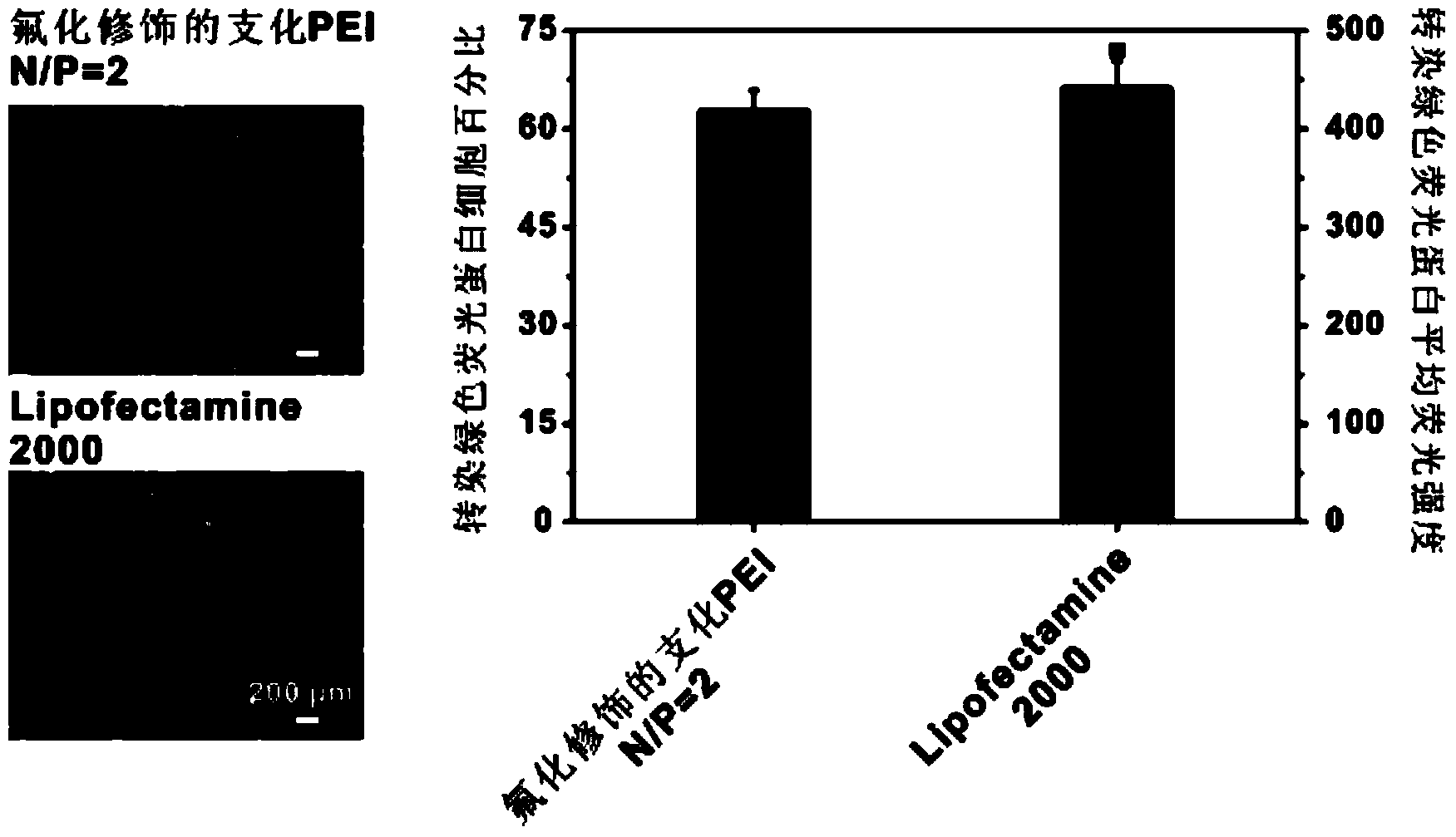
Fluorine-containing aliphatic chain-modified cationic polymer and application of fluorine-containing aliphatic chain-modified cationic polymer as gene carrier
A technology of cationic polymers and aliphatic chains, applied in the fields of polymer chemistry and biomaterials, can solve the problems of high toxicity of polyethylenimine and insufficient transfection efficiency, and achieve high gene transfection efficiency, low price, and high-efficiency gene transfection dyed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1: Preparation of 3-(1H, 1H, 5H octafluoropentyloxy)-1,2-epoxypropylene modified branched polyethyleneimine, wherein branched polyethyleneimine and 3-(1H, 1H , 5H octafluoropentyloxy)-1,2-propylene oxide molar ratio is 1:133.
[0092] Synthetic method: take 50 mg of branched polyethyleneimine macromolecules in 2 ml of aqueous solution, slowly dropwise add 3-(1H, 1H, 5H octafluoropentyloxy)-1,2-epoxypropylene in 2 ml of ethanol solution, after the dropwise addition, the reaction solution was stirred and reacted at room temperature for 48 hours, and after dialysis and freeze-drying, the appearance It is a branched polyethyleneimine polymer (fluorinated branched PEI) modified by surface fluorination of white powder.
[0093] In this embodiment, the cationic polymer modified by the fluorine-containing fatty chain has a structure as shown in formula (II) in claim 1, the cationic polymer is a branched polyethyleneimine with an average molecular weight of 25000 Da, an...
Embodiment 2
[0100] Example 2: Preparation of third-generation polypropyleneimine dendrimer modified by heptafluorobutyric acid, wherein the molar ratio of dendrimer to heptafluorobutyric anhydride is 1:10.
[0101] Synthesis method: Dissolve 70.5 mg of third-generation polypropyleneimine dendrimers in 2 ml of methanol solution, add 69.94 microliters of triethylamine dropwise to the methanol solution in which dendrimers are dissolved, and then add 1 ml of methanol solution containing 171.5 micrograms of heptafluorobutyric anhydride was added dropwise, and after the addition was completed, the reaction solution was stirred and reacted at room temperature for 12 hours to obtain a white powder product.
[0102] The ninhydrin method was used to characterize and detect the linking quantity of heptafluorobutyric acid in the synthetic product.
[0103] Specific implementation method: add 0.2 mg / ml G3PPI dendrimer solution with a volume of 0, 5, 10, 15, 20, 25, 30, 35, and 40 microliters into a 1....
Embodiment 3
[0120] Example 3: Preparation of the fourth generation polypropyleneimine dendrimer modified by heptafluorobutyric acid, wherein the molar ratio of dendrimer to heptafluorobutyric anhydride is 1:16.
[0121] Synthetic method: Dissolve 53.4 mg of the fourth-generation polypropyleneimine dendrimer in 2 ml of methanol solution, add 40.7 microliters of triethylamine dropwise to the methanol solution in which dendrimers are dissolved, and then add Add 1 ml of methanol solution of heptafluorobutyric anhydride with 99.7 mg dropwise. After the addition is completed, the reaction solution is stirred and reacted at room temperature for 12 hours to obtain the surface heptafluorobutyric acid-modified white powder. The fourth generation polypropylene imine dendrimer material.
[0122] The ninhydrin method was used to characterize and detect the gene transfection vector synthesized in this example: the ninhydrin method was used to characterize and detect the heptafluorobutyric acid modifica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com