Populations of hematopoietic progenitors and methods of enriching stem cells therefor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
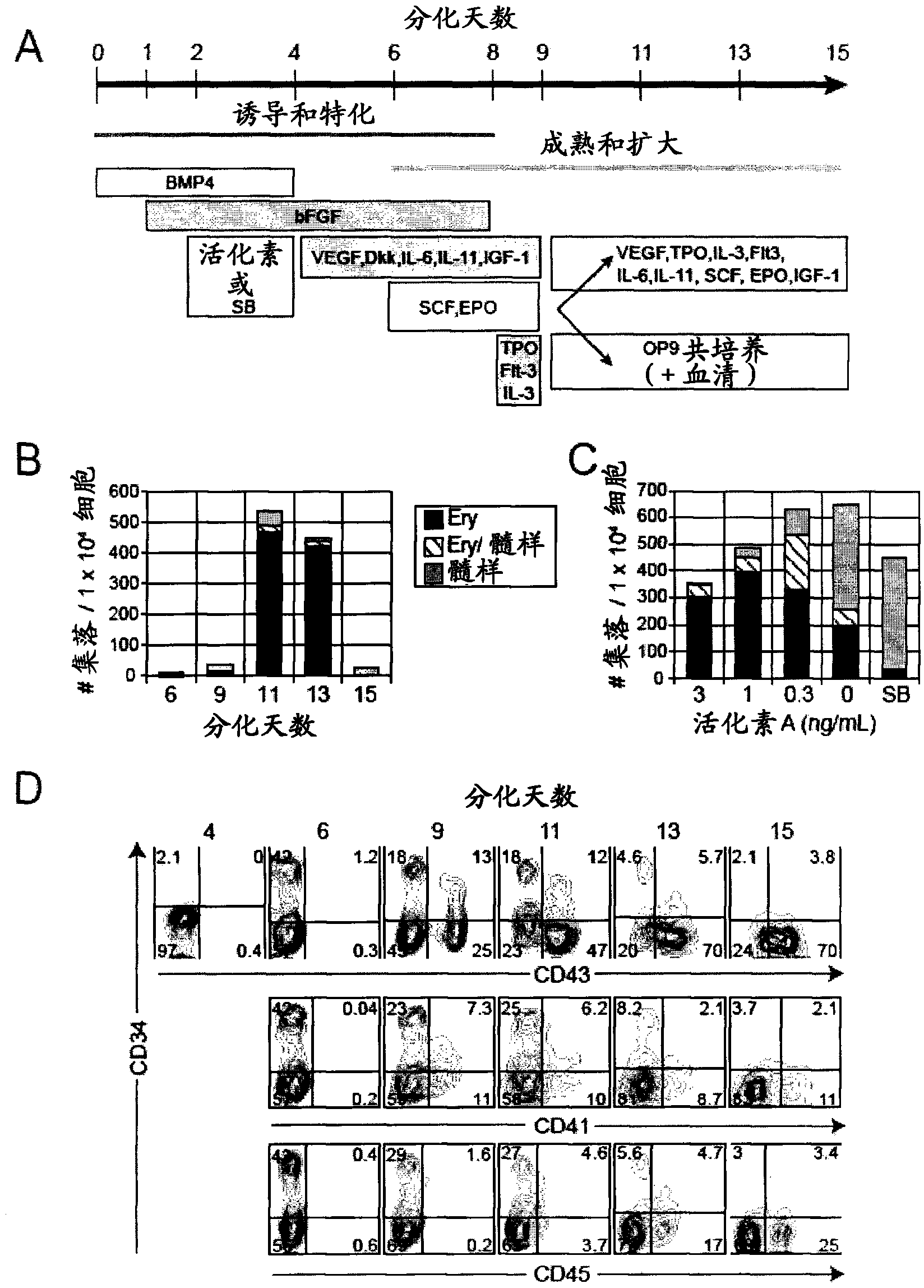
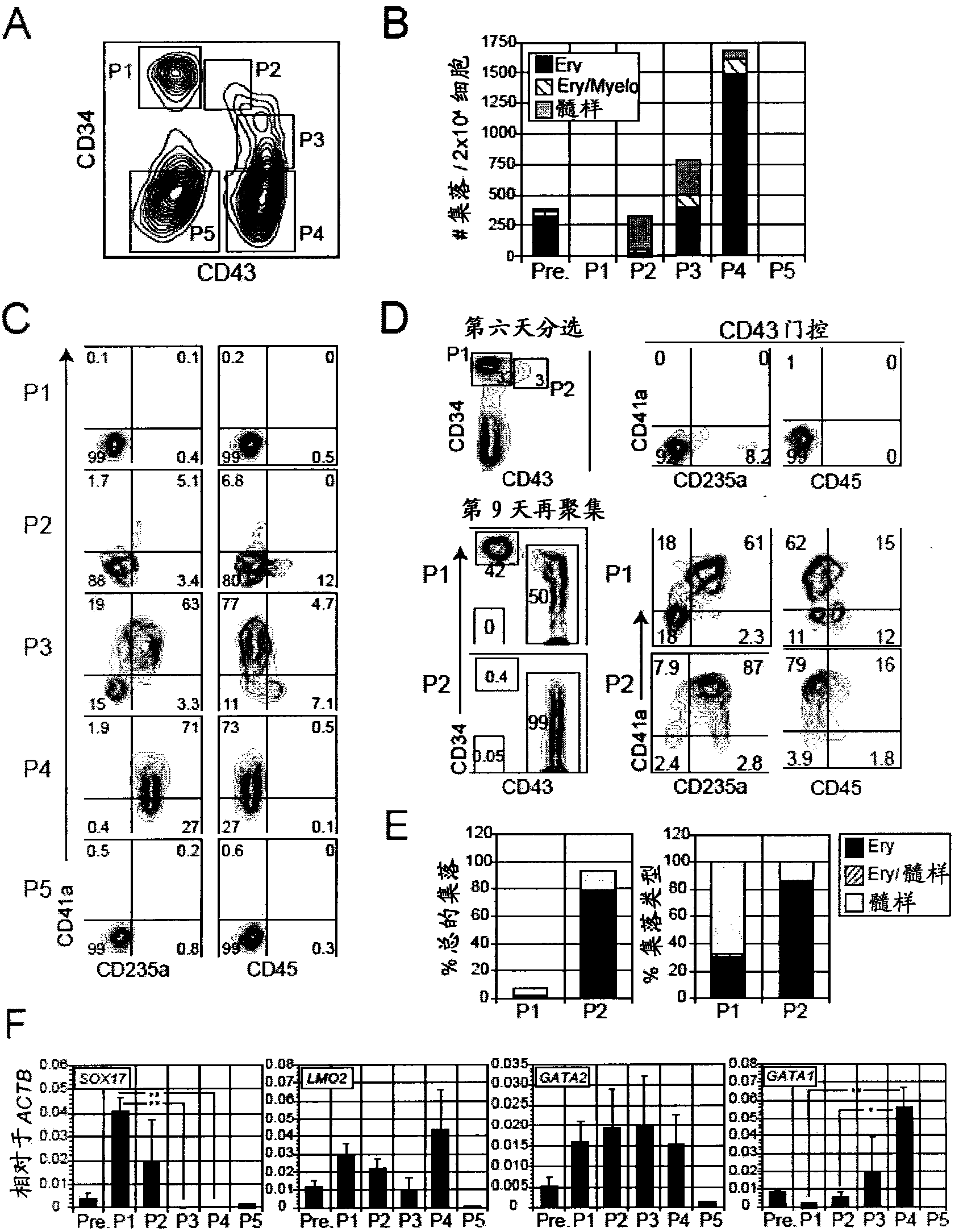
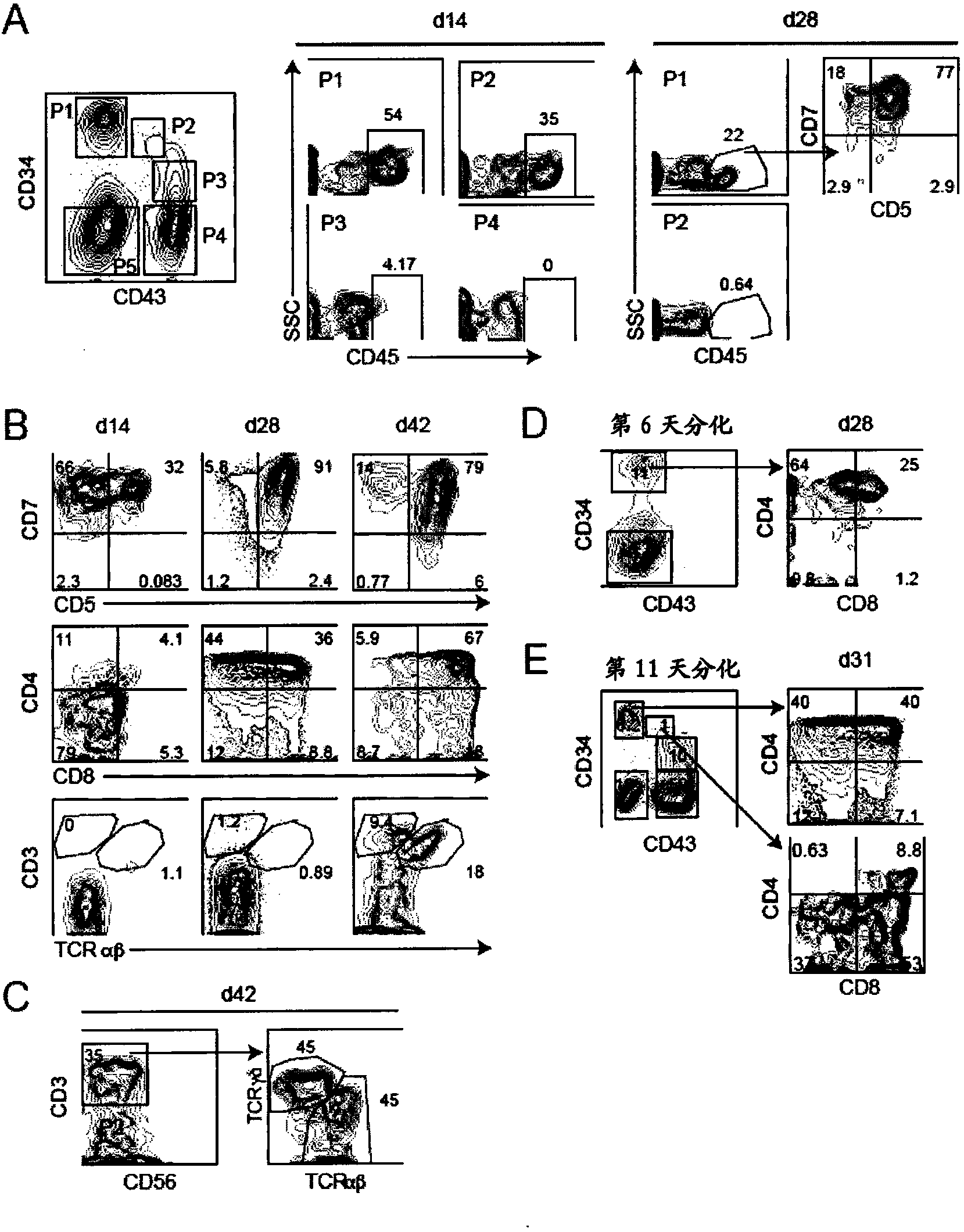
[0058] Materials and methods
[0059] Maintenance and differentiation of human ES and iPS cells
[0060] The hESC line H1 (Thomson et al., 1998) and a reprogrammed human iPS cell line (MSC-iPS1; (Park et al., 2008)) were used in this study. They were maintained on irradiated mouse embryonic fibroblasts in hESC medium as previously described (Kennedy et al., 2007). Cells were depleted of feeder cells by culturing on Matrigel (BD Biosciences, Bedford, MA) in hESC medium for 24 to 48 hours prior to differentiation. To generate EBs, hPSCs were treated with collagenase B (1 mg / ml; Roche, Indianapolis, IN) for 20 minutes, followed by a brief trypsin-EDTA (0.05%) step. Gently scrape the cells with a cell scraper to form small aggregates (10-20 cells). The aggregates were resuspended in supplemented with penicillin / streptomycin (10ng / ml), L-glutamine (2mM), ascorbic acid (1mM), thioglycerol (MTG, 4×10 -4 M; Sigma) and transferrin (150 μg / ml) in StemPro-34 (Invitrogen). BMP-4 (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com