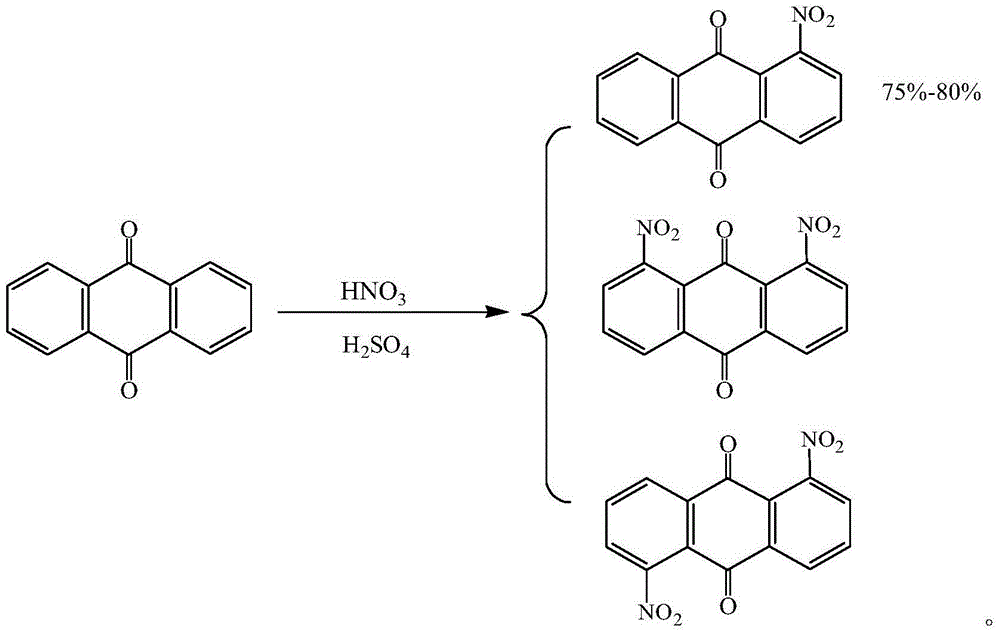
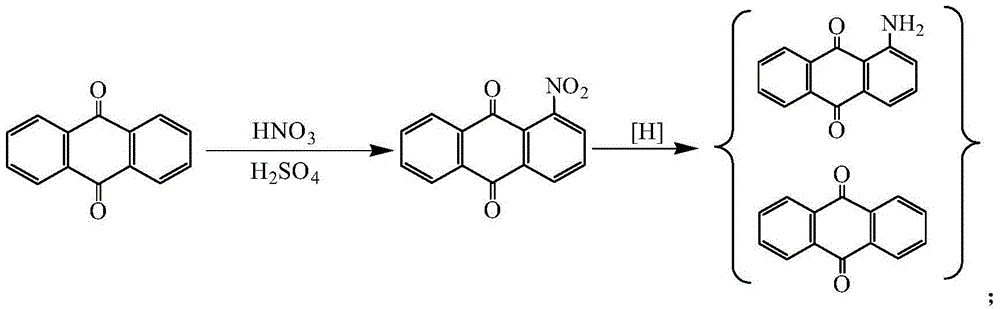
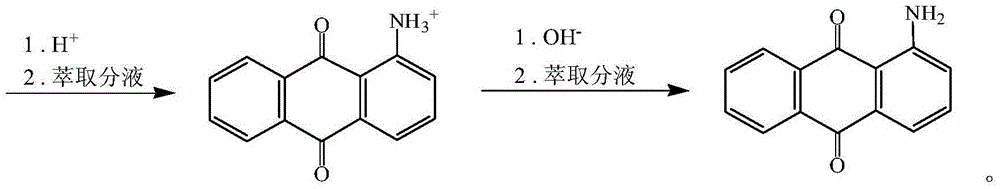
Method for synthesizing 1-aminoanthraquinone
A technique for the synthesis of aminoanthraquinones, which is applied in chemical instruments and methods, preparation of nitro compounds, preparation of organic compounds, etc., can solve the problems of large amount of solvent, low level of total yield, large energy consumption, etc., and achieve production The effect of low cost and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) In a 500ml four-necked flask, add 130ml of dichloroethane, 20ml of DMF, add 120g of anthraquinone, 1.2g of p-toluenesulfonic acid, and 24.08g of 98% nitric acid under stirring, and maintain the reaction temperature at 30°C ± 5°C, Slowly add 56g of oleum dropwise within 10 hours. After adding oleum, keep the temperature at 40°C for 6 hours, then add 80ml of water and adjust the pH to 5 with ammonia water, evaporate the solvent under reduced pressure, and recover dichloroethane and DMF. 140.26 g of the solid mixture was obtained. The 1-nitroanthraquinone content is 83.04%, and the anthraquinone content is 17.01%.
[0034] (2) Add the solid mixture obtained in the above (1) into a solution of 33.6g sodium hydrosulfide and 120g water in batches, keep it at 25°C for 3 hours, then raise the temperature to 90°C to 95°C and continue the reaction for 2 hours, filter and wash with water , to obtain 126.91 g of a solid mixture. The 1-aminoanthraquinone content is 81.09%, and...
Embodiment 2
[0037] (1) In a 500ml four-neck flask, add 80ml of xylene and 10ml of DMF, add 60g of anthraquinone, 0.5g of sodium p-toluenesulfonate under stirring, add 7.41g of 98% nitric acid, and maintain the reaction temperature at 30°C ± 5°C, 10 Slowly add 30g of oleum dropwise within 1 hour, after adding oleum, keep the temperature at 40°C for 8 hours, then add 50ml of water and adjust the pH to 5 with ammonia water, evaporate the solvent under reduced pressure, recover xylene and DMF, and obtain a solid mixture 70.37g. The 1-nitroanthraquinone content is 83.54%, and the anthraquinone content is 16.51%.
[0038] (2) Add the solid mixture obtained in the above (1) in batches to a solution of 25 g of sodium hydrosulfide and 100 g of water, maintain 25° C. for 2 hours, then raise the temperature to 90° C. to 95° C. and continue the reaction for 3 hours, filter, wash with water, 63.46 g of a solid mixture was obtained. The 1-aminoanthraquinone content is 81.10%, and the anthraquinone co...
Embodiment 3
[0041] (1) In a 500ml four-neck flask, add 120ml of chlorobenzene, 20ml of DMF, add 120g of anthraquinone, 0.6g of p-toluenesulfonate sodium, 0.5g of p-toluenesulfonic acid, add 33.34g of 98% nitric acid, and maintain the reaction temperature 30°C±5°C, slowly add 60g of oleum dropwise within 10 hours, after adding the oleum, keep the temperature at 40°C for 6 hours, then add 80ml of water and adjust the pH to 5 with 15% NaOH, evaporate the solvent under reduced pressure, Chlorobenzene and DMF were recovered to obtain 139.93 g of a solid mixture. The 1-nitroanthraquinone content is 83.98%, and the anthraquinone content is 16.01%.
[0042] (2) Add the solid mixture obtained in the above (1) in batches to a solution of 35.0 g of sodium hydrosulfide and 120 g of water, maintain 25 ° C for 2 hours, then raise the temperature to 90 ° C to 95 ° C and continue the reaction for 3 hours, filter and wash with water , to obtain 126.95 g of a solid mixture. The 1-aminoanthraquinone conte...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap