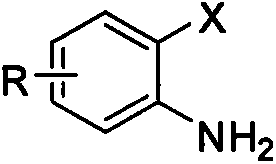
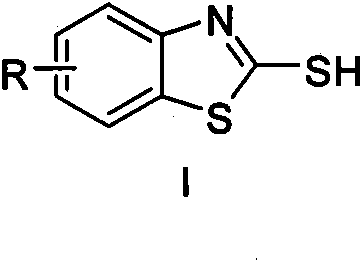
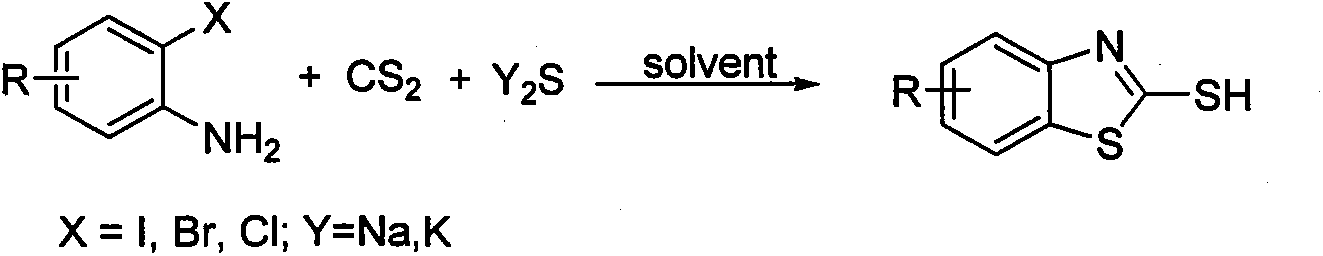Method for utilizing inorganic metal sulfide to promote reaction of carbon disulfide and 2-halogen phenylamine to synthesize 2-mercaptobenzothiazole
A synthetic method and inorganic metal technology, which is applied in the fields of medicine, industry and agriculture, can solve problems such as environmental pollution and complex processing, and achieve the effects of simple post-processing, mild reaction conditions, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1, using o-iodoaniline as raw material to synthesize 2-mercaptobenzothiazole (reaction temperature 130° C.)
[0016] In the reaction test tube, add 0.50mmol (0.1095g) of o-iodoaniline and 1.00mmol (0.2402g) of sodium sulfide nonahydrate, then add 2mL N, N-dimethylformamide and 1.50mmol ( 0.1142g) of carbon disulfide, stirred and reacted at 130°C for 12 hours. After the o-iodoaniline was completely reacted by TLC, the reaction solution was cooled to room temperature, added with 3 mL of 4N hydrochloric acid and stirred for 15 minutes, and then the reaction solution was extracted three times with dichloromethane. The organic phases were combined, dried over anhydrous magnesium sulfate for 2 hours, filtered to remove the desiccant, and finally the dichloromethane solvent was distilled off under reduced pressure to obtain a crude product. The crude product was separated by column chromatography (200-300 mesh silica gel), and gradient elution was carried out using ...
Embodiment 2
[0017] Example 2, using o-iodoaniline as raw material to synthesize 2-mercaptobenzothiazole (reaction temperature 90°C)
[0018] In the reaction test tube, add 0.50mmol (0.1095g) of o-iodoaniline and 1.00mmol (0.2402g) of sodium sulfide nonahydrate, then add 2mL N, N-dimethylformamide and 1.50mmol ( 0.1142g) of carbon disulfide, stirred and reacted at 90°C for 12 hours, after TLC detected that the o-iodoaniline had reacted completely, the reaction solution was cooled to room temperature, added 3mL of 4N hydrochloric acid and stirred for 15min, then the reaction solution was extracted three times with dichloromethane, The organic phases were combined, dried over anhydrous magnesium sulfate for 2 hours, filtered to remove the desiccant, and finally the dichloromethane solvent was distilled off under reduced pressure to obtain a crude product. The crude product was separated by column chromatography (200-300 mesh silica gel), and gradient elution was carried out using petroleum e...
Embodiment 3
[0019] Example 3, Synthesis of 2-mercaptobenzothiazole with o-iodoaniline as raw material (reaction temperature 110°C)
[0020] In the reaction test tube, add 0.50mmol (0.1095g) of o-iodoaniline and 1.00mmol (0.2402g) of sodium sulfide nonahydrate, then add 2mL N, N-dimethylformamide and 1.50mmol ( 0.1142g) of carbon disulfide, stirred and reacted at 110°C for 12 hours. After the o-iodoaniline was completely reacted by TLC, the reaction solution was cooled to room temperature, added with 3 mL of 4N hydrochloric acid and stirred for 15 minutes, and then the reaction solution was extracted three times with dichloromethane. The organic phases were combined, dried over anhydrous magnesium sulfate for 2 hours, filtered to remove the desiccant, and finally the dichloromethane solvent was distilled off under reduced pressure to obtain a crude product. The crude product was separated by column chromatography (200-300 mesh silica gel), and gradient elution was carried out using petrole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap