Gene expression diagnostic chip for acute exacerbation of chronic obstructive pulmonary disease
A chronic obstructive and acute exacerbation technology, applied in the field of biomedical detection, can solve problems such as identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 The process of screening 15 gene probes
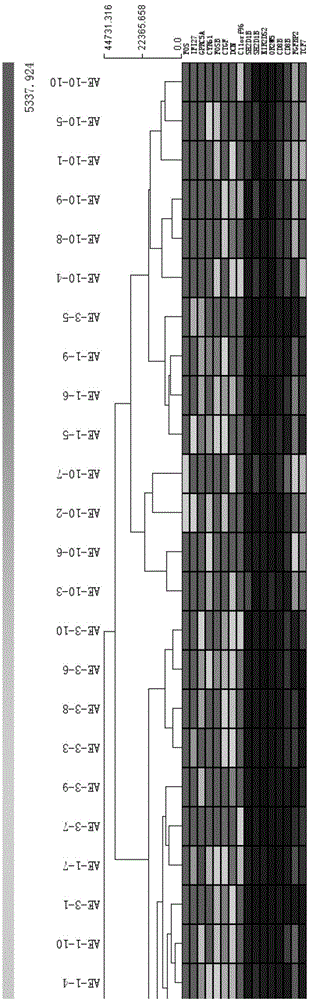
[0021] The peripheral blood of 10 patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD), 10 patients with stable chronic obstructive pulmonary disease (StCOPD) and 10 healthy people (Control) were collected. The expression of more than 45,000 genes in peripheral blood mononuclear cells (PBMC) was detected by gene expression profiling chip technology, and the patient groups with acute exacerbation of chronic obstructive pulmonary disease (AE-1) and day 3 (AE-1) were screened respectively. -3) Compared with the 10th day (AE-10)), the genes up-regulated or down-regulated in the stable patients with chronic obstructive pulmonary disease (StCOPD) and the healthy control group (Control) were compared, and then the common differences in different stages of acute exacerbation were screened again At the same time, they were matched with the clinical severity score (DESS), and finally the differentia...
Embodiment 2
[0028] Example 2 Design and manufacture of gene expression diagnostic chip for acute exacerbation of chronic obstructive pulmonary disease
[0029] Use silanized glass slides as the chip carrier, soak in 5% glutaraldehyde for 50min, ddH 2 O Ultrasonic cleaning 2 times, put in a dry place for later use.
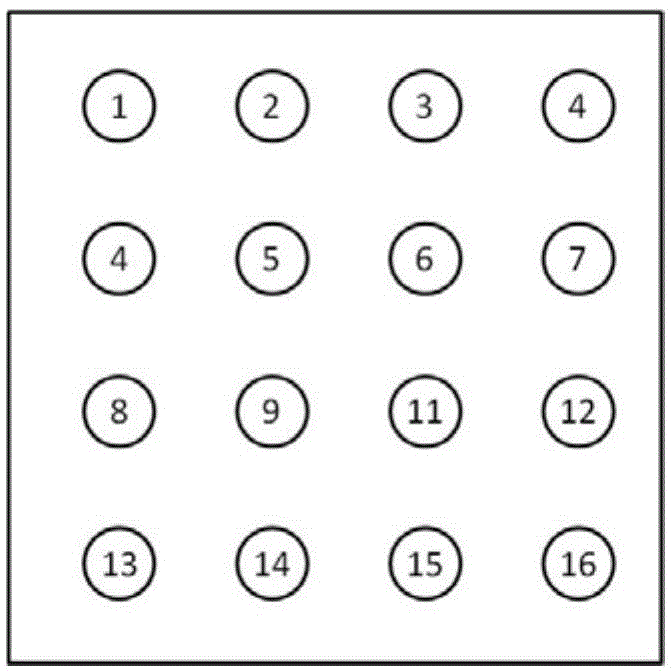
[0030] The probes corresponding to the genes in Table 4 and the probes corresponding to the positive internal reference probe ACTB were dissolved in 3×SSC respectively, and the concentration of the probes was 100 mM. The above 15 probe solutions and the blank control were dissolved according to figure 2 The array was spotted with a Spotting Apparatus Cartesian Tech PixSys 5500, and the spotting volume at each position was 1nl. After sample application, hydration at 37°C for 2h.
[0031] The probe sequences are shown in Table 4, of which No. 15 is a positive internal reference.
[0032] Table 4
[0033]
[0034]
[0035] When in use, first wash twice with 0.2% SDS, ...
Embodiment 3
[0036] Example 3, the application of gene expression diagnostic chip detection for acute exacerbation of chronic obstructive pulmonary disease
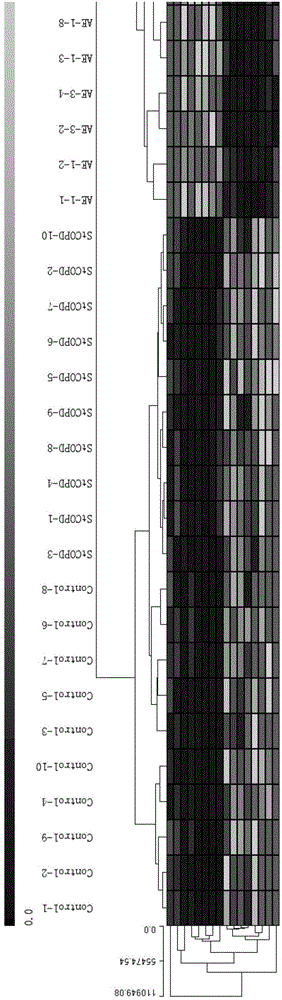
[0037] Sixty samples of peripheral blood (20 from stable COPD, 30 from AECOPD and 10 from healthy controls) were collected.
[0038] Separation of peripheral blood mononuclear cells: 1ml of blood + 1ml of normal saline to make a cell suspension, slowly drop the cell suspension on 1ml of lymphocyte separation medium, centrifuge at 2500rpm for 20min, at this time 5 layers are formed in the centrifuge tube: the top is plasma , between the plasma layer and the lymphocyte separation medium is PBMC, between the lymphocyte separation liquid layer and the bottom erythrocyte layer is the granulocyte layer; collect the mononuclear cells at the interface between the plasma layer and the lymphocyte separation medium, and aspirate all the PBMC as much as possible Add physiological saline to wash twice, centrifuge at 1500rpm for 5min, discard the u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com