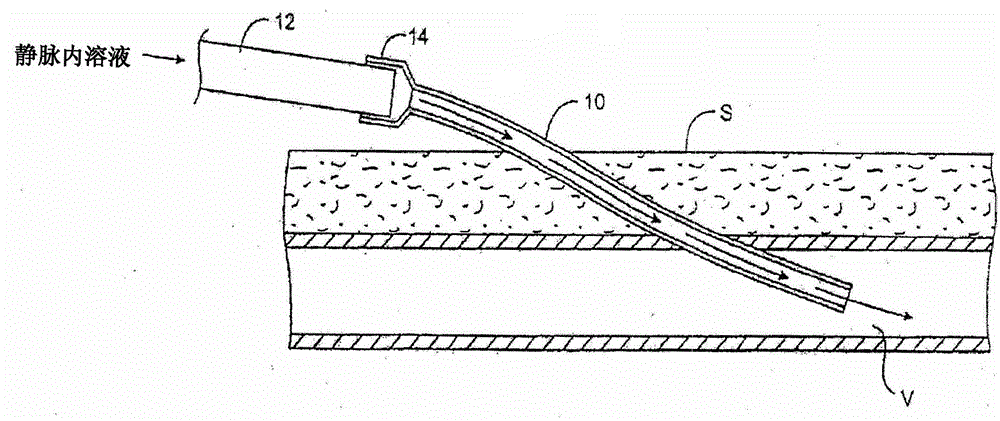
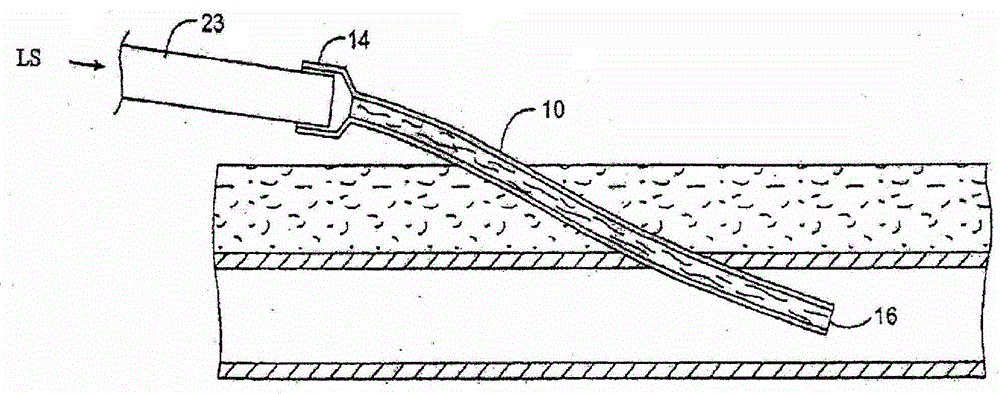
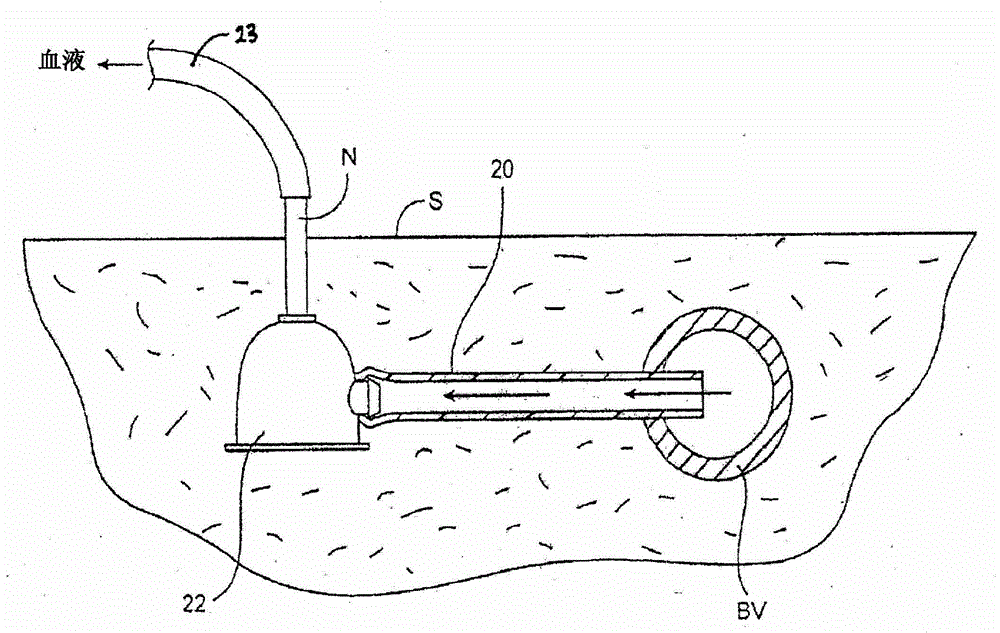
Transdermal venous access locking solutions
A solution, growth-inhibiting technology, used in disinfection, coating, chemistry, etc., to solve problems such as lack of anticoagulant characteristics, access obstruction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0082] Example 1 Evaluation of Microbial Growth Inhibitory Solutions
[0083] The following studies provide antimicrobial / antifungal testing of formulated microbial growth inhibitory solutions of the present invention.
[0084] Methicillin-resistant Staphylococcus aureus ("MRSA")
[0085] Stock cultures of S. aureus were maintained as frozen stock cultures at -70°C until use. The bacterial test uses Staphylococcus aureus isolated from human blood. Cell suspensions were prepared from frozen stock cultures and grown in Tryptic Soy Broth ("TSB") to obtain approximately 1 x 10 8 MRSA in colony forming units ("CFU"). Use plate counts to confirm the final concentration of the inoculum solution.
[0086] Bacterial Test - For the test, 100 ul of MRSA cell suspension was inoculated into 9.9 ml of each test solution to obtain approximately 1 x 10 6 Final cell concentration of CFU / ml. This represents 1×10 8 1:100 dilution of CFU / ml starter culture; calculate initial cell concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com