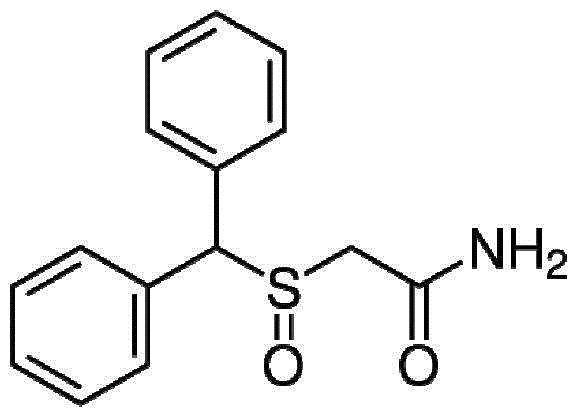
Use of modafinil in the treatment of cocaine addicts
A technology for cocaine and addiction, applied in the direction of medical preparations containing active ingredients, organic active ingredients, pill delivery, etc., can solve the problem of continuous effect beyond the patient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] It has been presented that modafinil in CO 2 has acceptable solubility.
[0031] The next step consists of spraying the dissolved active ingredient on an inert carrier and then investigating the particle size of the obtained granules and the crystalline form of the same granules.
[0032] First, different experiments were carried out using anhydrous lactose as an inert carrier.
[0033] Then modify the different parameters:
[0034] changing the inert carrier (mannitol instead of anhydrous lactose),
[0035] Increase loading level to 30% active ingredient loading,
[0036] Using S modafinil,
[0037] In addition to CO 2 external supercritical solvents.
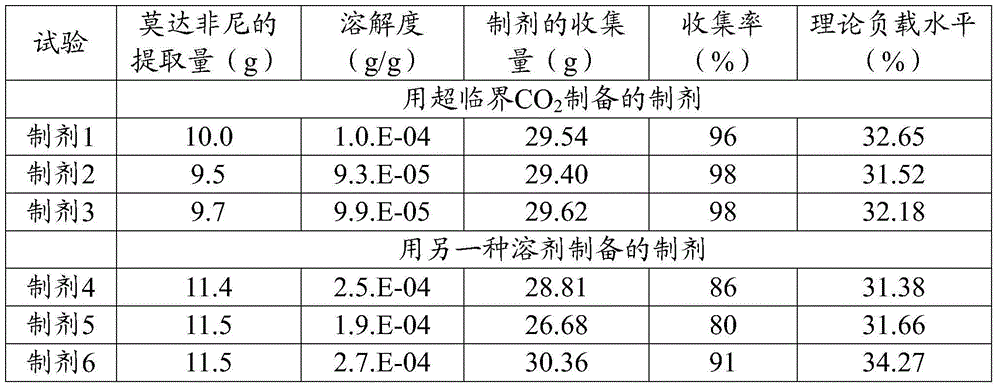
[0038] Trials using S modafinil and mannitol are summarized in the table below.
[0039]
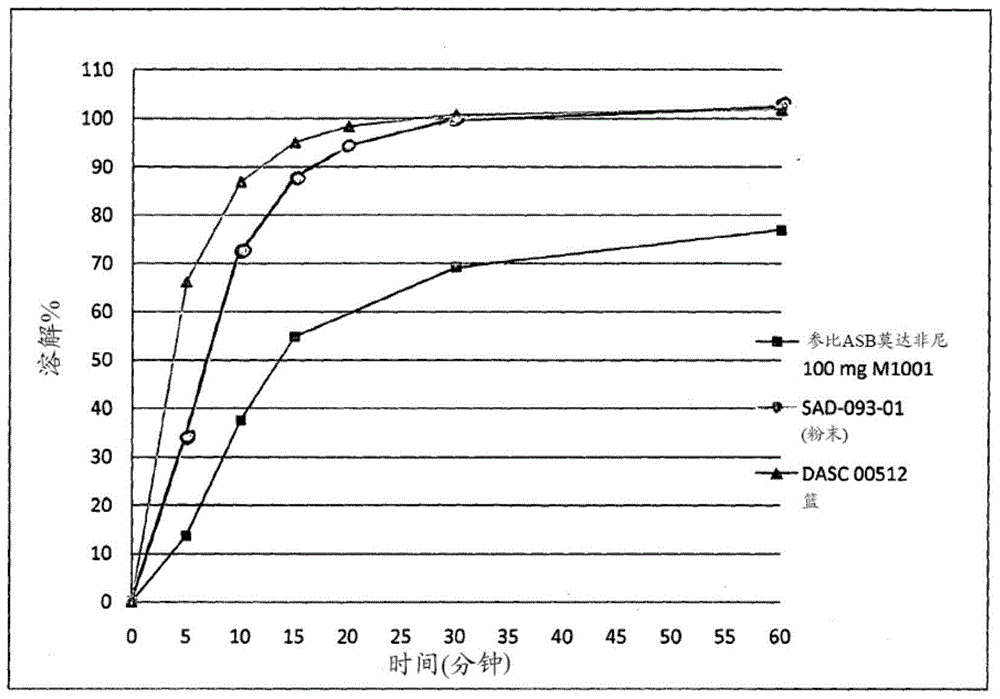
[0040] The previously obtained formulation was formulated in order to obtain tablets of S modafinil at a dose of 2 mg for administration to rats during pharmacokinetic studies.
[0041] The method used is as follows:
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com