Application of O-(tetrahydropyrrole) ethyl derivative of cleistanthus sumatranus ketone in preparation of medicine for treating or preventing renal fibrosis
A technology of tetrahydropyrrolyl and ethyl derivatives, which can be used in pharmaceutical combinations, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve problems such as low safety and high toxicity, and achieve good resistance to renal fibrosis. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
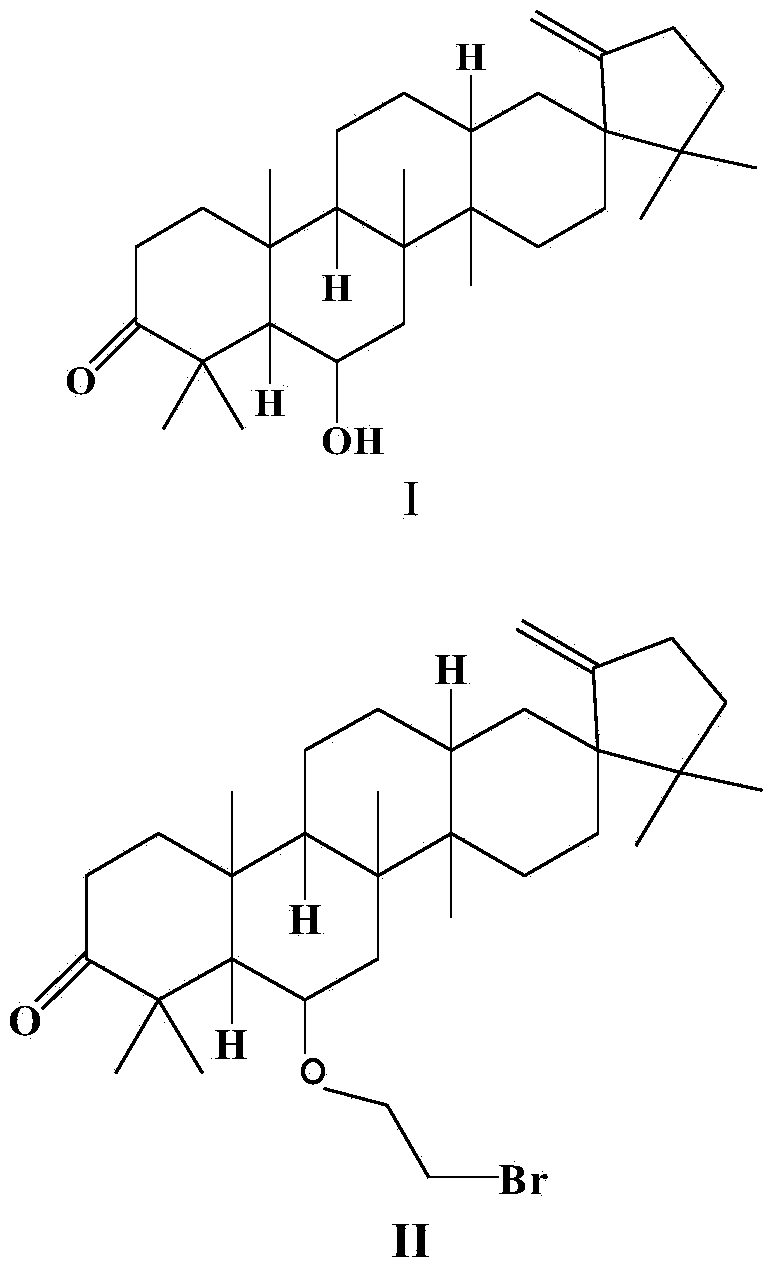
[0017] Example 1 Preparation of Compound Cleistanone
[0018] The preparation method of the compound Cleistanone (I) refers to the literature published by Van Trinh Thi Thanh et al. , pages 4108–4111, August 2011).
[0019]
Embodiment 2
[0020] Example 2 Synthesis of O-Bromoethyl Derivatives (II) of Cleistanone
[0021] Compound I (440 mg, 1.00 mmol) was dissolved in 10 mL of benzene, and tetrabutylammonium bromide (TBAB) (0.04 g), 1,2-dibromoethane (3.760 g, 20.00 mmol) and 6 mL of 50% sodium hydroxide solution. The mixture was stirred at 25 °C for 24 h. After 24 hours, the reaction solution was poured into ice water, extracted twice with dichloromethane immediately, and the organic phase solutions were combined. Then the organic phase solution was washed with water and saturated brine three times successively, then dried with anhydrous sodium sulfate, and finally concentrated under reduced pressure to remove the solvent to obtain a crude product. The crude product was purified by silica gel column chromatography (mobile phase: petroleum ether / acetone=100:1, v / v), and the yellow concentrated elution band was collected to obtain compound II as a yellow solid (344 mg, 63%).
[0022] 1H NMR (500MHz, DMSO-d 6...
Embodiment 3
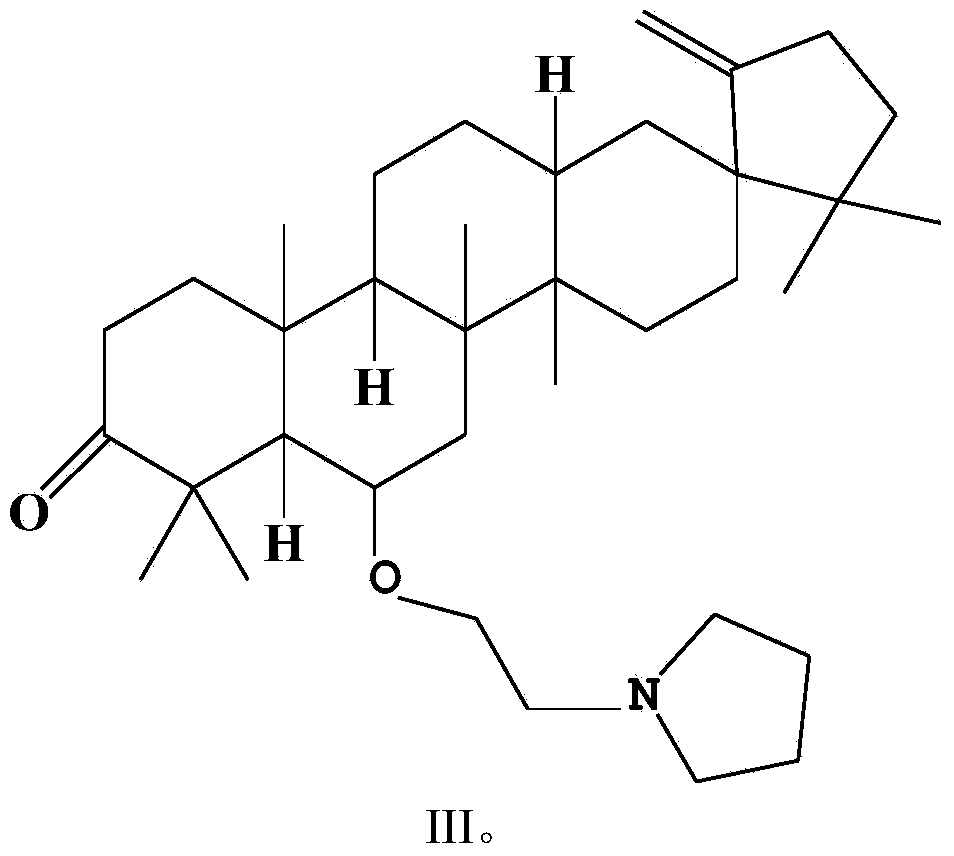
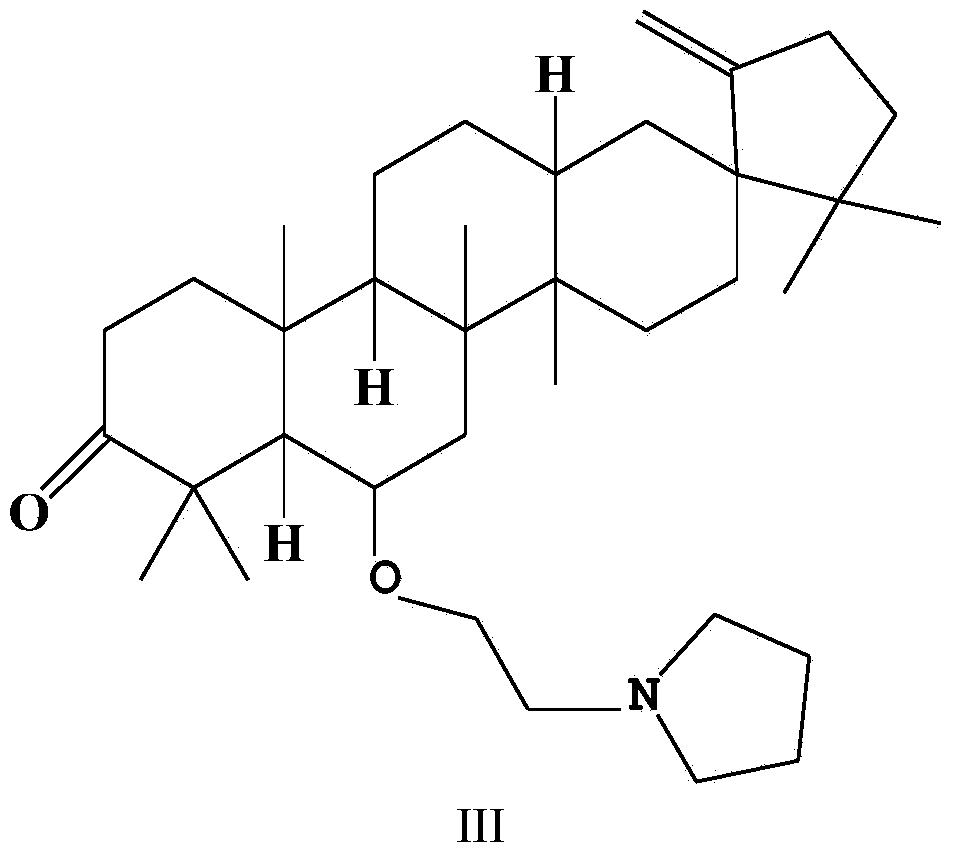
[0026] Example 3 Synthesis of O-(tetrahydropyrrolyl) ethyl derivatives (III) of cleistanone Cleistanone
[0027] Compound II (273 mg, 0.5 mmol) was dissolved in 20 mL of acetonitrile, anhydrous potassium carbonate (345 mg, 2.5 mmol), potassium iodide (84 mg, 0.5 mmol) and pyrrolidine (1420 mg, 20 mmol) were added thereto, and the mixture was heated to reflux for 8 h. After the reaction was completed, the reaction solution was poured into ice water, extracted three times with an equal amount of dichloromethane, and the organic phases were combined. The combined organic phases were successively washed with water and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to remove the solvent to obtain a crude product. The crude product was purified by silica gel column chromatography (mobile phase: petroleum ether / acetone=100:1, v / v), and the light yellow concentrated elution band was collected to obtain the O-(tetrahydropyrrolyl)ethyl deri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com