Medical dressing with sodium carboxymethylcellulose/polylactic acid sponge protection layer
A technology of sodium carboxymethyl cellulose and polylactic acid, applied in dressings, viscous dressings, medical science and other directions, can solve the problems of poor use compliance, poor fixation performance, strong sense of restraint, etc. Healing, good fixation effect, improved fixation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0008] The preparation method of sodium carboxymethyl cellulose / polylactic acid sponge, adopts the aqueous solution of sodium carboxymethyl cellulose of 5% concentration by weight and the α-pyrrolidone solution of polylactic acid of 2% concentration by weight and then undergoes vacuum freezing It is dry prepared, and the volume ratio of sodium carboxymethyl cellulose solution and α-pyrrolidone solution of polylactic acid is 1:1.
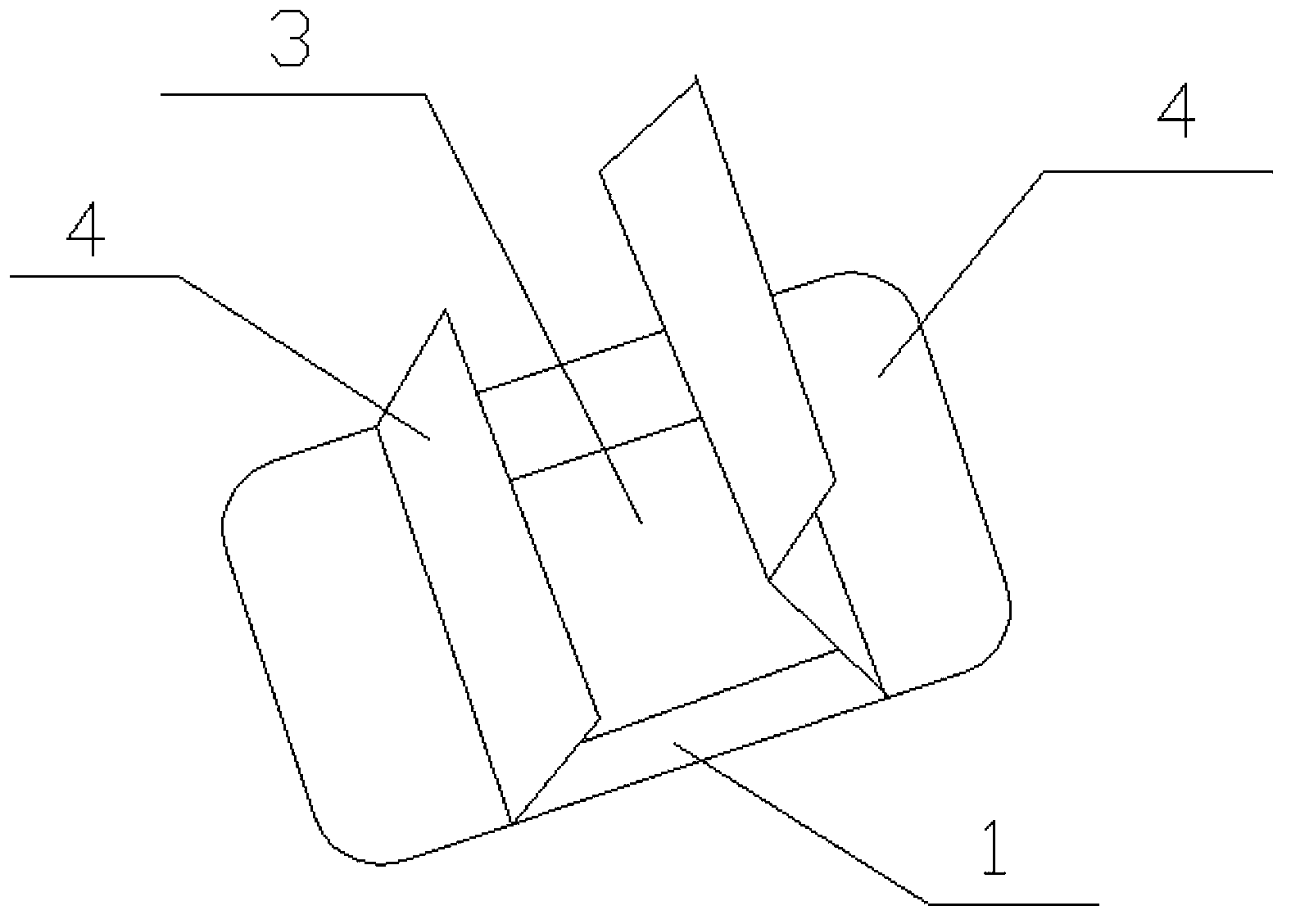
[0009] Such as figure 1 In the dressing shown, the base fabric is rectangular, the base fabric 1 has a viscose layer 2, the middle part of the base fabric 1 is pasted with a protective layer 3 on the viscose layer 2, and the protective layer is sodium carboxymethyl cellulose / polylactic acid sponge with a thickness of 1.5 mm, the protective layer 3 is covered with a release paper 4 , and the outer edge of the release paper 4 is fixed on the corresponding exposed part around the base cloth 1 .
[0010] The outer side of the base cloth 1 adopts the med...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com