Conjugated polymer, preparation method and applications thereof
A technology of conjugated polymers and compounds, applied in semiconductor/solid-state device manufacturing, organic chemistry, electric solid-state devices, etc., can solve problems such as low photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
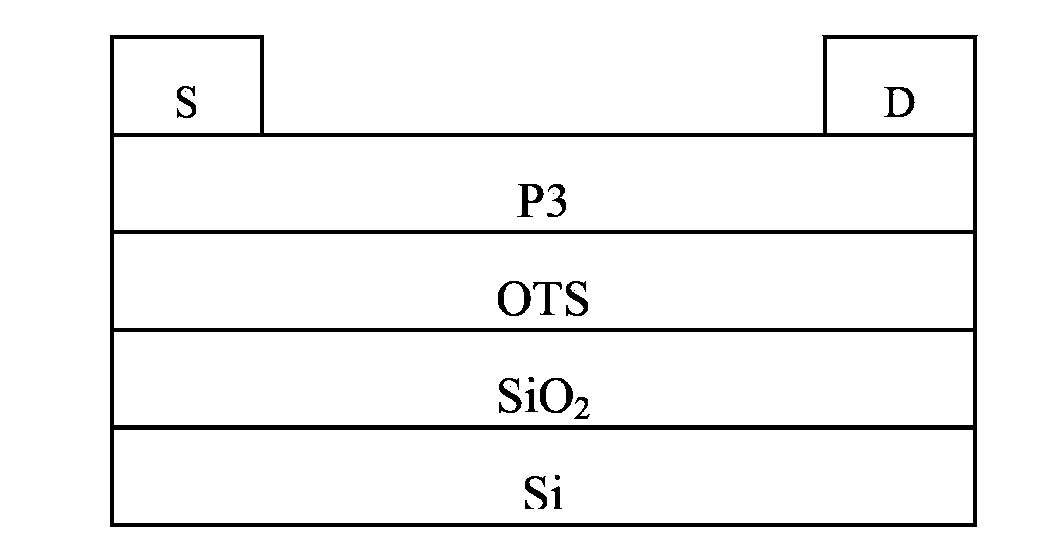
Image
Examples
preparation example Construction
[0036] The present invention also includes the preparation method to above-mentioned conjugated polymer, and this method comprises the following steps:
[0037] a) provide the following compounds A and B;
[0038] Compound A is
[0039] Compound B is
[0040] b) Under an inert gas atmosphere, add the compound A and the compound B into the organic solvent at a molar ratio of 1.1 to 1.5:1, remove the residual oxygen by bubbling, then quickly add the catalyst, and remove the residual oxygen by bubbling again , carry out the Stille coupling reaction at 60-120°C for 24-72 hours, separate and purify to obtain the conjugated polymer (P);
[0041]
[0042] Among them, R is H or C 1 ~C 12 Alkyl group, n is a natural number between 5 and 60.
[0043] The catalyst may be tetrakis(triphenylphosphine)palladium or bis(triphenylphosphine)palladium dichloride; or
[0044]The catalyst can also be a mixture of organic palladium and organic phosphine ligands, the molar ratio of the ...
Embodiment 1
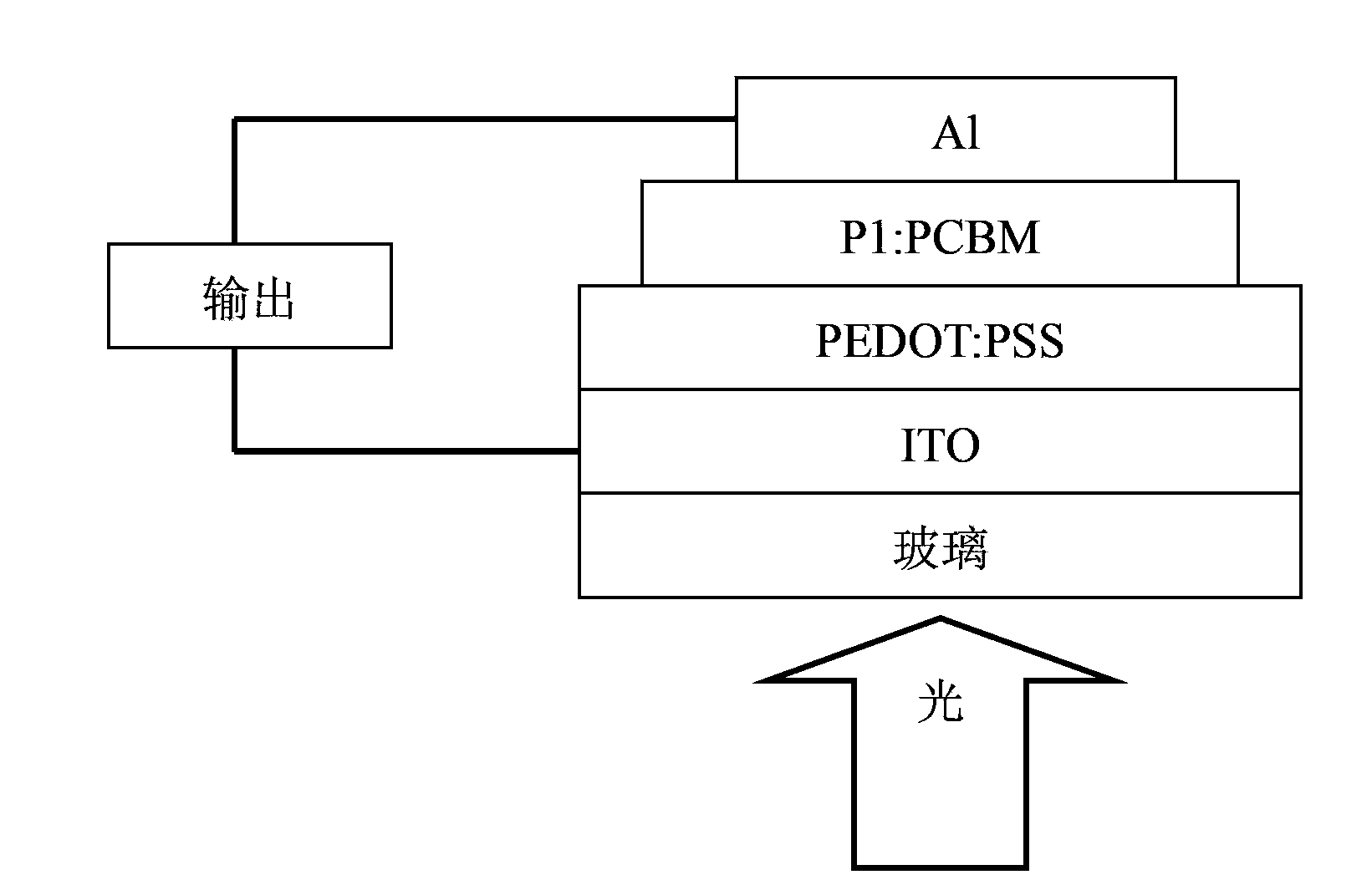
[0058] The conjugated polymer disclosed in this example is specifically compound P1, and its structural formula is as follows:
[0059]
[0060] 1. Preparation of compound A1
[0061] 1) Under nitrogen atmosphere, add 812mg (2.0mmol) 5,8-dibromodithieno[3',2':3,4;2'',3'':5,6] to the reaction flask Benzo[1,2-c][1,2,5]thiadiazole (c1), 1.15g (2.2mmol) 3,6-di(dodecyl)thieno[3,2-b]thiophene -2-boronic acid (d1), 0.64g (6.0mmol) sodium carbonate, 0.23mg (0.0002mmol) Pd(PPh 3 ) 4 , 60ml of dry tetrahydrofuran. The reaction mixture was stirred and refluxed at 60° C. for 24 h, then cooled to room temperature, and the reaction solution was poured into distilled water to quench the reaction. Then extract with dichloromethane, combine the organic phases, wash with saturated brine, dry and filter, remove a large amount of organic solvent by rotary evaporation, and obtain compound (e1) by separation and purification on a silica gel column, the eluent is ethyl acetate / petroleum ether...
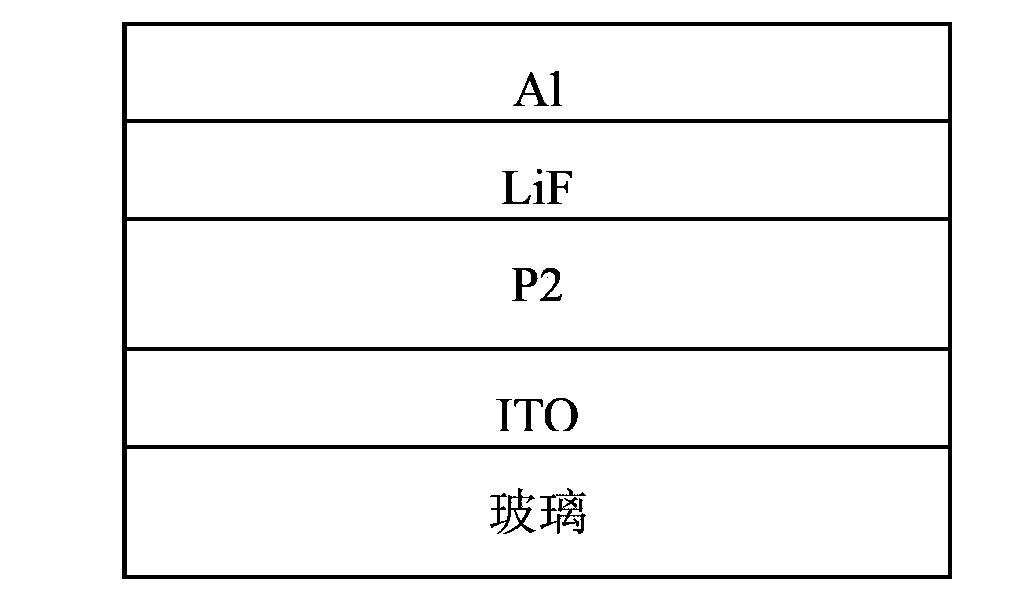
Embodiment 2
[0070] The conjugated polymer disclosed in this example is specifically compound P2, and its structural formula is as follows:
[0071]
[0072] One, the preparation of compound A2:
[0073] 1) Under nitrogen atmosphere, add 812mg (2.0mmol) 5,8-dibromodithieno[3',2':3,4;2'',3'':5,6] to the reaction flask Benzo[1,2-c][1,2,5]thiadiazole (c2), 0.44g (2.4mmol) Thieno[3,2-b]thiophene-2-boronic acid (d2), 1.38g( 10.0mmol) of potassium carbonate, 115.6mg (0.1mmol) of Pd (PPh 3 ) 4 , 70 ml dry DMF. The reaction mixture was stirred and reacted at 78° C. for 36 h, then cooled to room temperature, and the reaction solution was poured into distilled water to quench the reaction. Then extract with dichloromethane, combine the organic phases, wash with saturated brine, dry and filter, remove a large amount of organic solvent by rotary evaporation, and obtain compound (e2) by separation and purification on a silica gel column, the eluent is ethyl acetate / petroleum ether=1 / 5 (v / v), t...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap