A kind of method utilizing microreactor to prepare caprolactam by α-amino-ε-caprolactam
A technology of caprolactam and micro-reactor, applied in the field of chemical synthesis and technology, to achieve the effects of easy disassembly, cost reduction, and large industrial application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
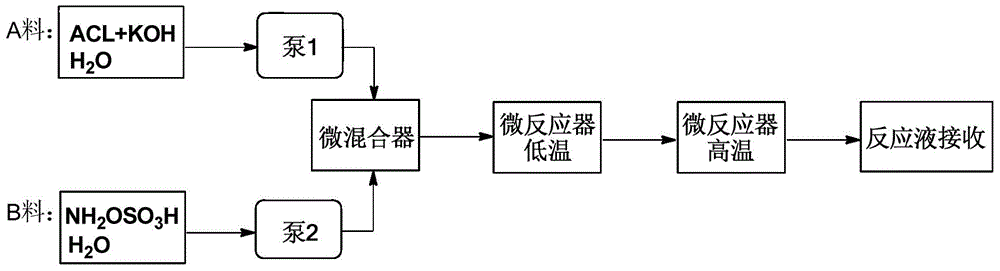
[0023] according to figure 1 The flow chart, the α-amino-ε-caprolactam lye (wherein, alkali is potassium hydroxide, concentration is 2wt%) and the hydroxylamine-O-sulfonic acid solution that concentration is 6wt% as mobile phase respectively with concentration is 2wt% Pump into the mixer to mix, control the flow rate to keep the pH at 10, and the molar ratio of α-amino-ε-caprolactam to hydroxylamine-O-sulfonic acid to potassium hydroxide is 1:2:4. The material passes through the 0°C low-temperature reactor with a residence time of 10 minutes, and directly flows into the 90°C high-temperature reactor with a residence time of 10 minutes. After the completion, the materials were concentrated, extracted with dichloromethane, and the organic extracts were combined, and the solvent was distilled off under reduced pressure to obtain the product caprolactam, with a yield of 80% and a selectivity of 94%.
Embodiment 2
[0025] according to figure 1 The flow chart, the concentration is the α-amino-ε-caprolactam lye (wherein, alkali is potassium hydroxide, concentration is 3wt%) and the concentration is the hydroxylamine-O-sulfonic acid solution of 3wt% as mobile phase respectively with the concentration of 1wt% Pump into the mixer to mix, control the flow rate to keep the pH at 10, and the molar ratio of α-amino-ε-caprolactam to hydroxylamine-O-sulfonic acid to potassium hydroxide is 1:2.5:5. The material passes through the 0°C low-temperature reactor with a residence time of 15 minutes, and directly flows into the 90°C high-temperature reactor with a residence time of 10 minutes. After the completion, the materials were concentrated, extracted with dichloromethane, and the organic extracts were combined, and the solvent was distilled off under reduced pressure to obtain the product caprolactam, with a yield of 85% and a selectivity of 91%.
Embodiment 3
[0027] according to figure 1The flow chart, the α-amino-ε-caprolactam lye (wherein, alkali is potassium hydroxide, concentration is 2wt%) and the hydroxylamine-O-sulfonic acid solution that concentration is 6wt% as mobile phase respectively with concentration is 2wt% Pump into the mixer for mixing, control the flow rate to keep the pH at 12, and the molar ratio of α-amino-ε-caprolactam to hydroxylamine-O-sulfonic acid to potassium hydroxide is 1:2:4. The material passes through the -5°C low-temperature reactor with a residence time of 5 minutes, and directly flows into the 95°C high-temperature reactor with a residence time of 10 minutes. After the completion, the materials were concentrated, extracted with ethyl acetate, and the organic extracts were combined, and the solvent was distilled off under reduced pressure to obtain the product caprolactam, with a yield of 81% and a selectivity of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com