Application of ruxolitinib in the preparation of drugs for the treatment of M2 type acute myeloid leukemia
A technology for acute myeloid leukemia, applied in the field of application of Ruxolitinib in the preparation of drugs for the treatment of M2 type acute myeloid leukemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
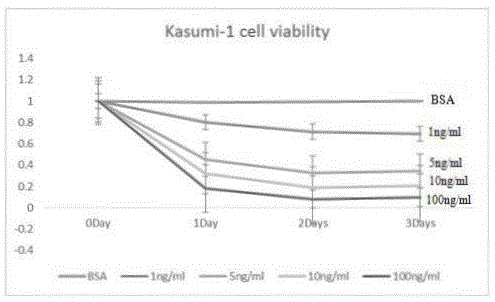
[0048] Example 1 In vitro effects of Ruxolitinib and / or rsTRAIL on Kasumi-1 cells
[0049] 1 Materials and methods
[0050] 1.1 Material
[0051] 1.1.1 Cell lines
[0052] Kasumi-1 cells: donated by Professor Zhu Jiang from the Institute of Blood Research of Ruijin Hospital, Shanghai, using a medium containing 10% FBS + RPMI1640 at 37°C, 5% CO 2 , Cultivate in an incubator under saturated humidity, and take log-phase cells for experiment.
[0053] 1.1.2 Main reagents
[0054] Table 1
[0055]
[0056] 1.1.3 Main instruments
[0057] Table 2
[0058]
[0059] 1.2 method
[0060] 1.2.1 Cell culture
[0061] Cell culture conditions: Kasumi-1 cells were inoculated in RPMI1640 medium containing 10% FBS under aseptic conditions, at 37℃, 5% CO 2 Culture in an incubator.
[0062] Cell passage: After the cells are centrifuged at low speed, the supernatant is discarded, the cells are resuspended in 3-5ml medium, and transferred to the culture flask to continue the culture.
[0063] Cryopreservation of ce...
Embodiment 2
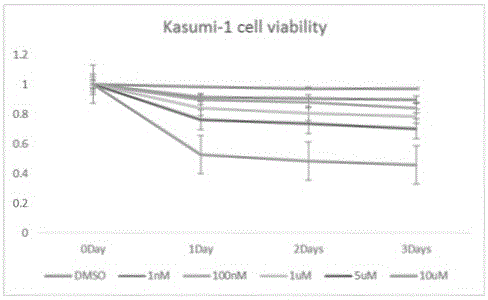
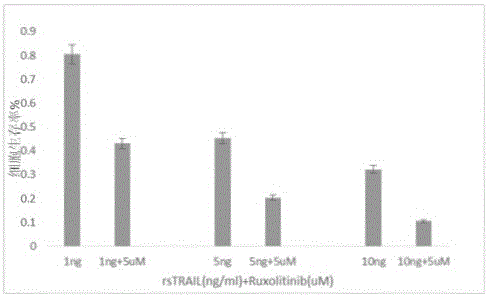
[0114] Example 2 Ruxolitinib enhances the mechanism of rsTRAIL on Kasumi-1 cell apoptosis in vitro
[0115] 1. Materials and methods
[0116] 1.1 Material
[0117] 1.1.1 Primer
[0118] According to the human TRAIL, DR4, DR5, DcR1, DcR2, JAK1, JAK2, and mRNA sequences provided by the NCBI database, with β-actin as an internal reference, primers were designed with the software Primer5.0, and synthesized by Shanghai Shenggong Biological Co., Ltd.:
[0119] DR4 primer sequence:
[0120] Forward Primer: 5’aaatgggtacaacaaaactggac3’
[0121] Reverse Primer: 5’gagcctgtgccatcttctaagt3’
[0122] DR5 primer sequence:
[0123] Forward Primer:5’agacttggtgccctttgactc3’
[0124] Reverse Primer: 5’cggttttgttgacccactttat3’
[0125] DcR1 primer sequence:
[0126] Forward Primer: 5’attacaccaacgcttccaaca3’
[0127] Reverse Primer: 5’catctctggggagttttcattc3’
[0128] DcR2 primer sequence:
[0129] Forward Primer: 5’gtgtgtcagtgtgaaaaaggaag3’
[0130] Reverse Primer: 5’aggtagtgatagggagaggcaag3’
[0131] TRAIL primer seq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com