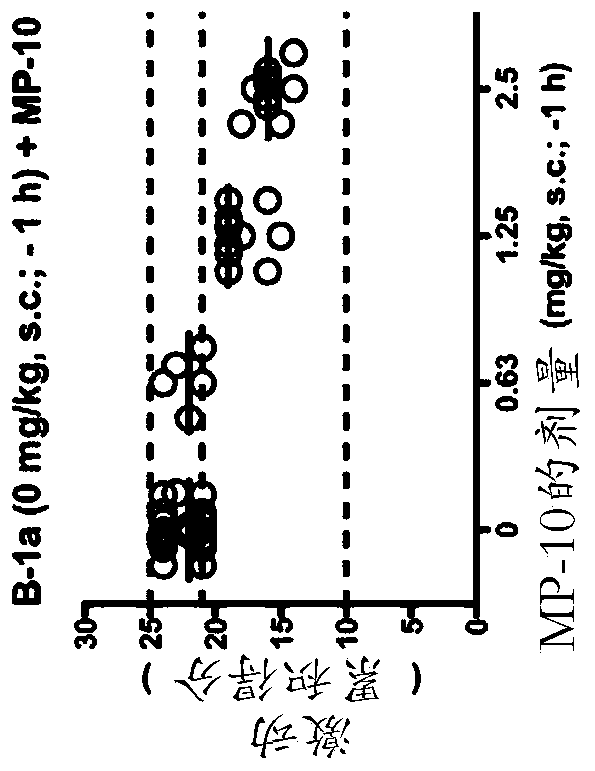
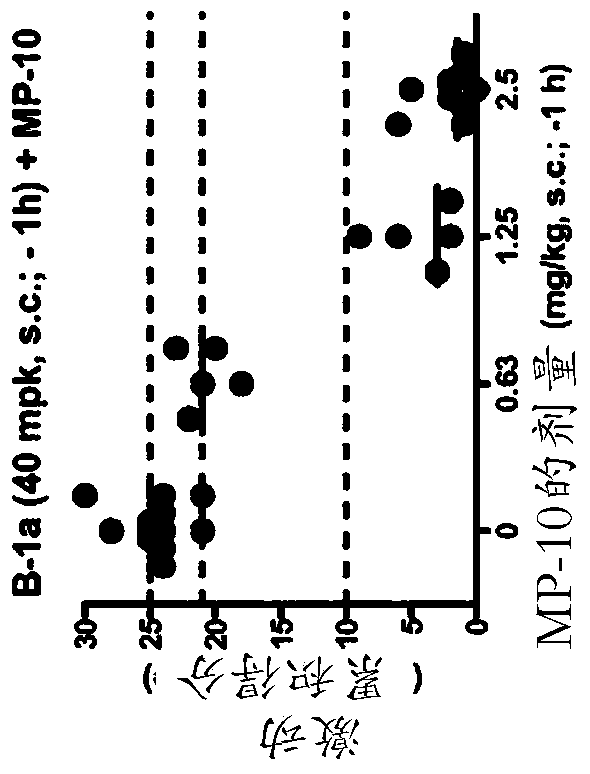
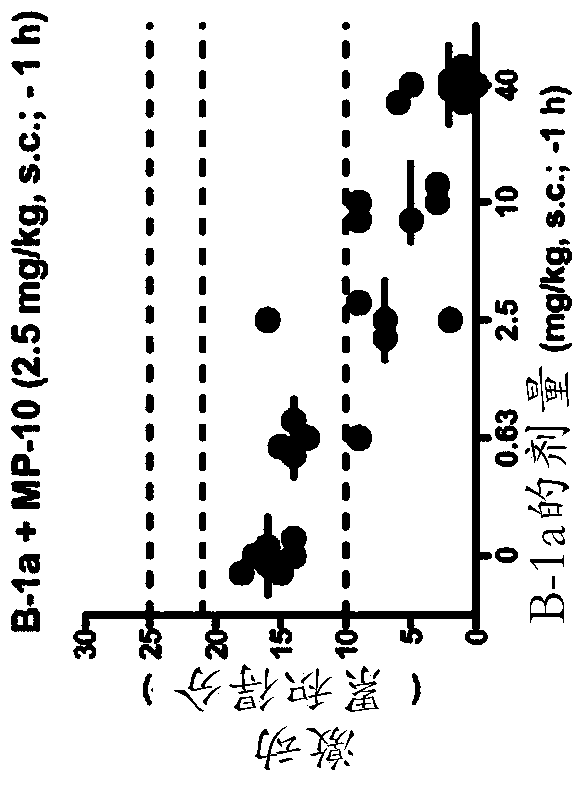
For use in the treatment of neurological disorders or metabolic combinations used in obstacles
A technology of PDE10 and inhibitors, applied in the field of phosphodiesterase 2 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0471] Examples 1a and 1b
[0472] 1-(5-Butoxypyridin-3-yl)-4-methyl-8-(morpholin-4-ylmethyl)[1,2,4]triazol[4,3-a]quinoxa Phenoline hydrochloride (B-1a) and oxalate (B-1b)
[0473]
[0474] .2HCl(B-1a) or .x C 2 h 2 o 4 (B-1b)
[0475] Formation of B-1a
[0476] To intermediate compound I-5 (7.5g, 18.19mmol) in THF / H 2 Add Pd(AcO) to a solution in O (10:1, 180 mL) 2 (0.12g, 0.54mmol), XantPhos (0.52g, 1.09mmol), Cs 2 CO 3 (23.88 g, 72.76 mmol) and intermediate compound 1-20 (4.51 g, 21.82 mmol). The reaction mixture was closed in a sealed tube and stirred at room temperature for 10 min and then at 114 °C for 45 min. Then, the crude mixture was washed with EtOAc and H 2 Diluted with O, the organic layer was separated and dried (MgSO 4 ), filtered and the solvent was concentrated in vacuo. The crude mixture was purified by chromatography (silica, MeOH in DCM, 0 / 100 to 2 / 98), the desired fractions were collected and the solvent was concentrated in vacuo to give ...
example 2
[0481] 1-(5-Butoxy-2-chlorophenyl)-4-methyl-8-(morpholin-4-ylmethyl)[1,2,4]triazole-[4,3-a] Quinoxaline hydrochloride (B-2)
[0482]
[0483] B-2 was synthesized as previously described for the synthesis of final compound B-1a. Starting from I-11a (0.2 g, 0.45 mmol) and intermediate I-20, the final compound B-2 (0.03 g, 14%) was obtained. 1 HNMR (300MHz, DMSO-d 6 )δppm 0.93(t, J=7.4Hz, 3H), 1.44(sxt, J=7.3Hz, 2H), 1.73(quin, J=6.9Hz, 2H), 2.93(br.s., 1H), 2.97( s,3H),3.19(br.s.,1H),3.77(br.s.,2H),3.92(br.s.,2H),3.98-4.14(m,2H),4.31(br.s. ,2H),5.76(s,2H),7.25(br.s,1H),7.33-7.50(m,2H),7.73(d,J=8.8Hz,1H),7.96(br.s.,1H) , 8.16 (d, J=8.1 Hz, 1H), 11.31 (br.s., 1H).
example 3
[0485] 1-(2-Chlorophenyl)-4-methyl-8-(morpholin-4-ylmethyl)[1,2,4]triazol[4,3-a]-quinoxaline (B- 3)
[0486]
[0487] To a stirred solution of Intermediate 1-8 (2.3 g, 7.12 mmol) dissolved in DCE (50 mL) was added morpholine (1.37 mL, 15.67 mmol) and the mixture was heated at 80 °C for 15 min under microwave irradiation (with The reaction was divided into three batches). Sodium triacetoxyborohydride (1.81 g, 8.55 mmol) was then added in portions and the mixture was heated again under the same conditions as previously described for 20 min. The mixture was then treated with H 2 O quenched and extracted with DCM. The organic layer was separated and dried (Na 2 SO 4 ), filtered and evaporated the solvent in vacuo. The crude compound was purified by chromatography (silica, MeOH in EtOAC, 2 / 98 to 10 / 90), the desired fractions were collected and the solvent was evaporated to give the final compound as a light yellow solid B-3 (1.6 g, 57%), this compound was further washed w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com