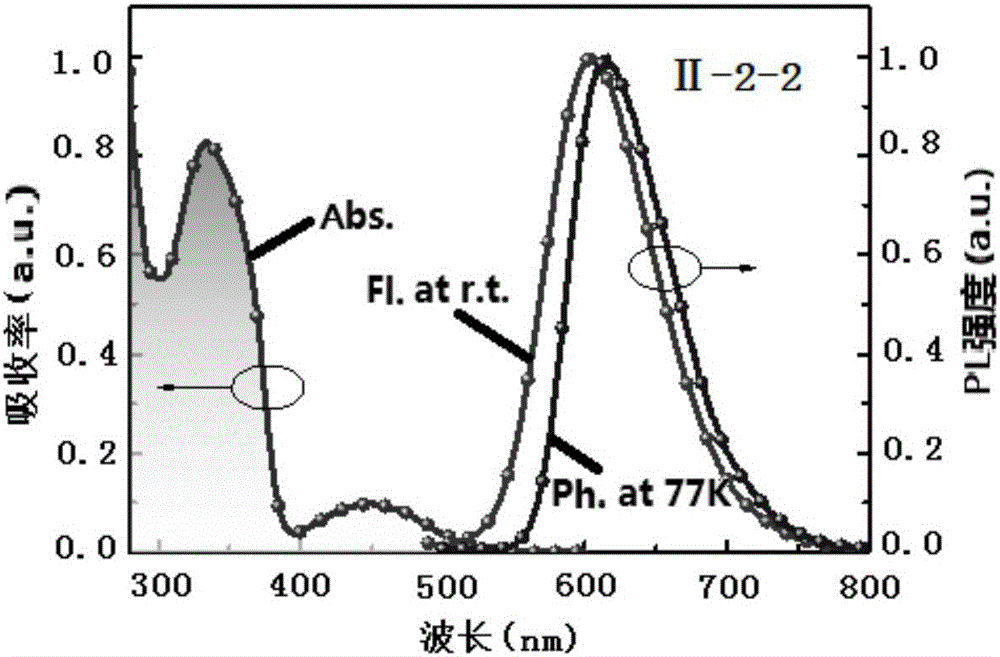
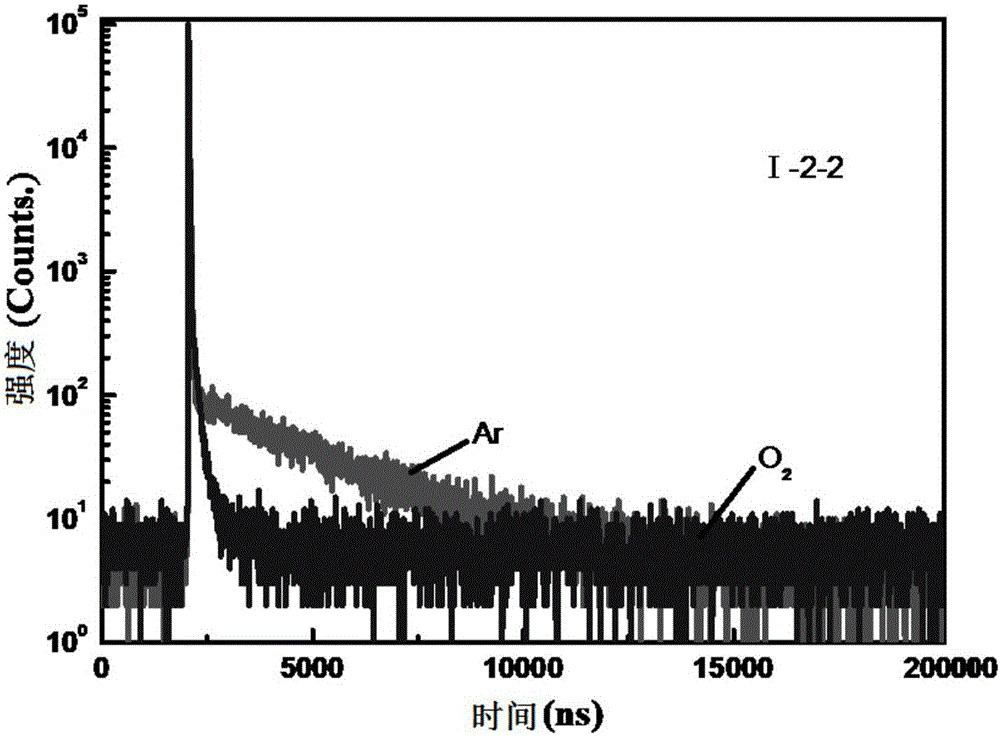
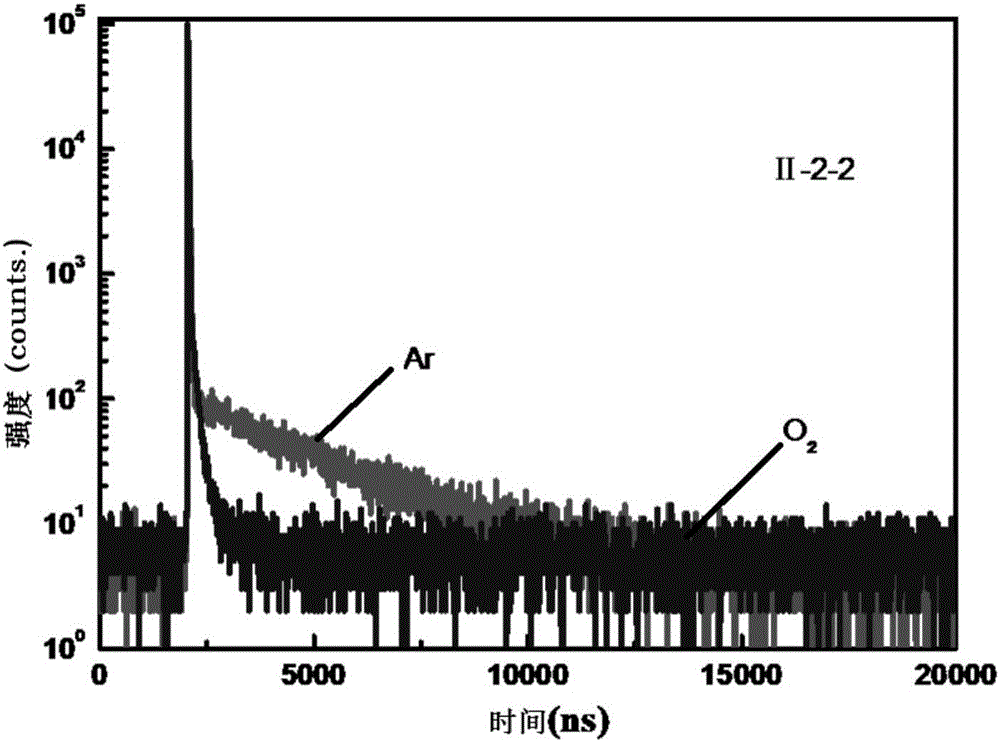
Quinoxaline unit containing organic thermally activated delayed fluorescent material and application thereof
A thermally induced delayed fluorescence, quinoxaline technology, applied in luminescent materials, organic chemistry, electrical components, etc., can solve the problems of expensive heavy metal materials, need to improve the stability, affecting practical use, etc., to achieve low attenuation, high-efficiency electroplating Luminous performance, high stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: the synthesis of important intermediate
[0060] The structures of intermediates ①~⑩ are as follows:
[0061]
[0062] (1) Synthesis of intermediate ①:
[0063]
[0064] 4-Fluoro-1,2-phenylenediamine (0.68g, 5.40mmol) and 4,4′-dibromobenzil (2.00g, 5.43mmol) were dissolved in toluene-acetic acid consisting of 20mL toluene and 30mL acetic acid solution, stirred evenly and refluxed for 8 hours, after the reaction was completed, cooled to room temperature, then poured the reaction solution into water, first extracted with dichloromethane, then dichloromethane / petroleum ether (v / v=2:3) Silica gel column chromatography separation was carried out as eluent, and drying was performed after rotary evaporation to obtain intermediate ① (2.10 g, yield rate 84%), which was a white powder.
[0065] (2) Synthesis of intermediate ②:
[0066] Select 4,5-difluoro-1,2-phenylenediamine and 4,4'-dibromobenzil as raw materials, refer to the synthesis steps and solvents...
Embodiment 2
[0084] Embodiment 2: Preparation of compound Ⅰ-1-1
[0085]
[0086] Intermediate ① (1.2g, 2.62mmol), phenothiazine (1.25g, 6.28mmol), sodium tert-butoxide (0.63g, 6.56mmol), palladium acetate (0.025g, 0.11mmol), tri-tert-butylphosphine Add tetrafluoroborate (0.095g, 0.33mmol) and 45mL of toluene into a 100mL round bottom flask together, reflux at 110°C for 48 hours, then quench the reaction with 10mL saturated aqueous sodium chloride solution, extract with dichloromethane first, then Dry over anhydrous sodium sulfate, and finally use dichloromethane / petroleum ether (v / v=2:3) as the eluent for silica gel column chromatography. The product is a yellow powder with a yield of 98%. Elemental Analysis Theoretical Value C 44 h 27 FN 4 S 2 (%): C 76.06, H 3.92, N 8.06; found values: C 76.15, H 4.01, N 8.11.
Embodiment 3
[0087] Embodiment 3: the preparation of compound Ⅰ-2-2
[0088]
[0089] Intermediate ② (1.2g, 2.52mmol), phenoxazine (1.06g, 5.79mmol), sodium tert-butoxide (0.58g, 6.04mmol), palladium acetate (0.023g, 0.10mmol), tri-tert-butylphosphine Add tetrafluoroborate (0.088g, 0.30mmol) and 40mL toluene into a 100mL round-bottomed flask, reflux at 110°C for 48 hours, then quench the reaction with 10mL saturated aqueous sodium chloride solution, extract with dichloromethane, then Dry over anhydrous sodium sulfate, and finally use dichloromethane / petroleum ether (v / v=2:3) as the eluent for silica gel column chromatography. The product is a yellow powder with a yield of 95%. Elemental Analysis Theoretical Value C 44 h 26 f 2 N 4 S 2 (%): C 76.64, H 3.85, N 8.23; found values: C 76.59, H 3.84, N 8.24.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com