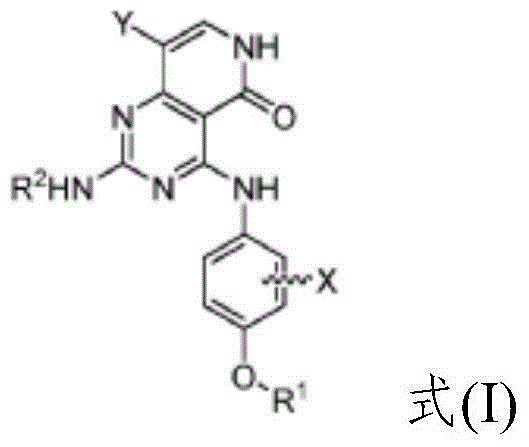
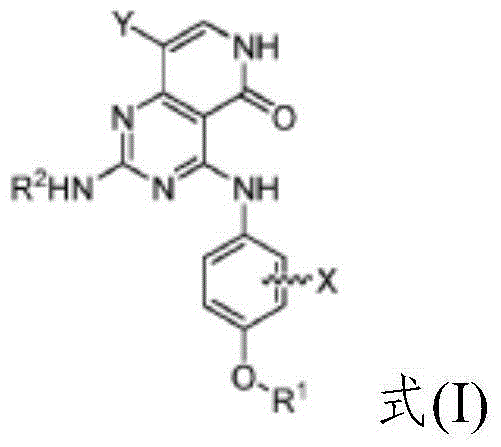
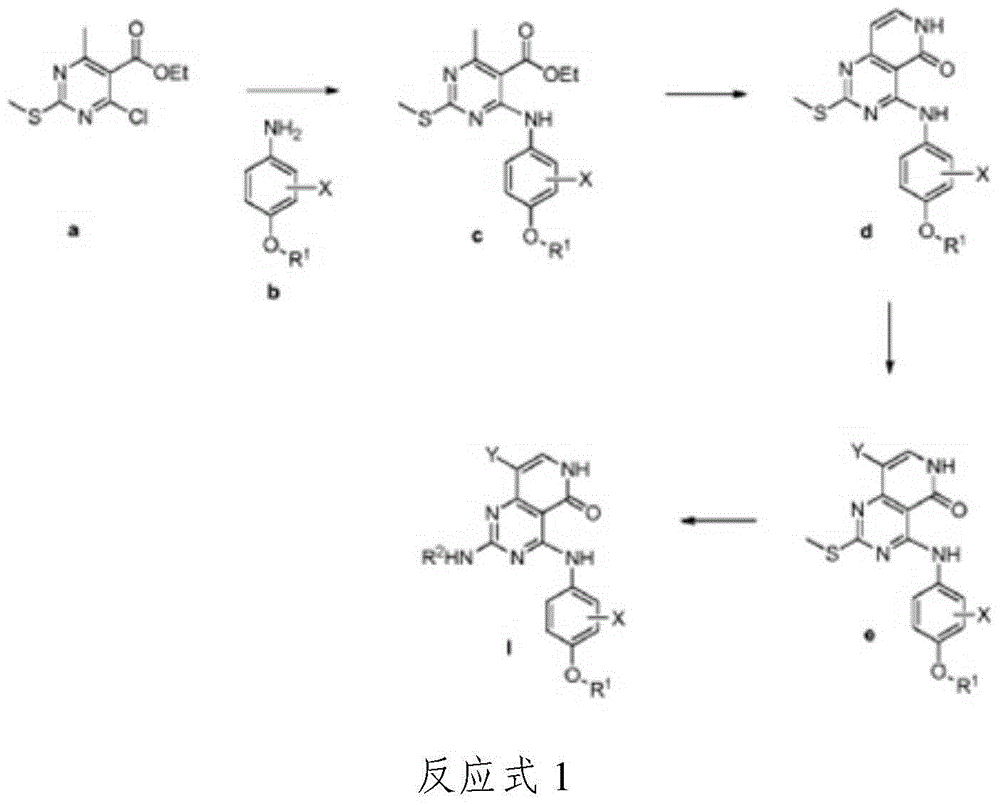
Substituted pyridopyrimidine compounds and their use as FLT3 inhibitors
A compound, C1-C3 technology, applied in the field of substituted pyridopyrimidine compounds and their use as FLT3 inhibitors, which can solve problems such as expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0111] The following examples illustrate the preparation of the compounds of formula (I) of the present invention and further illustrate the present invention by these examples. The examples are for illustrative purposes only and are not intended and should not be construed as limiting the invention in any way. Those skilled in the art will recognize that changes and modifications can be made without altering the scope of the invention.
[0112] Liquid chromatography-mass spectrometry (LC-MS) method:
[0113] 1. The sample was run at a flow rate of 1.5 mL / min on an Agilent Technologies 6120 MSD system with a Zorbax Eclipse XDB-C18 (3.5 μ) inverted column (4.6×50 mm) running at room temperature.
[0114] 2. The mobile phase uses solvent A (water / 0.1% formic acid) and solvent B (acetonitrile / 0.1% formic acid): 95% / 5% to 0% / 100% (A / B) for 5 minutes.
[0115] 3. Record mass spectra (m / z) using electrospray ionization (ESI).
[0116] 4. Ionization data is rounded to the nearest ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com