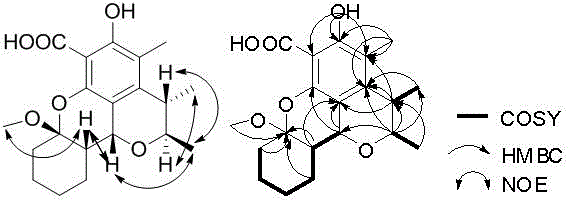
Citrinin compound penicitrinol N derived from penicillium citrinum as well as preparation method and application thereof
A compound, the technology of Penicillium citrinum, is applied in the field of citrinin compounds to achieve the effect of significant anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
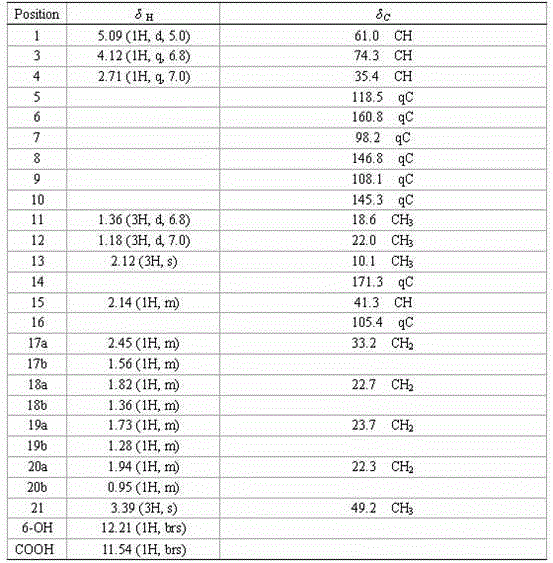
[0018] Example 1 Fermentation production and separation and purification of the compound
[0019] 1 Fermentation production
[0020] Fermentation culture of producing bacteria: Take Penicillium citrinum ( Penicillium citrinum ) IBPT-5 (has been deposited in the China Type Culture Collection on December 25, 2013, address: Wuhan University, Wuhan, deposit number: CCTCC NO: M 2013713) appropriate amount, inoculated on the PDA solid slant medium at 28 Cultivate in an incubator at ℃ for 4 days.
[0021] Take the penicillium citrinum ( Penicillium citrinum ) An appropriate amount of IBPT-5 is inoculated into 400mL culture solution [Composition of culture solution (g / L): mannitol 20.0, yeast extract 3.0, maltose 20.0, monosodium glutamate 10.0, glucose 10.0, KH 2 PO 4 0.5, MgSO 4 0.3, NaCl 30.0 constant volume] 1000mL Erlenmeyer flask, 28 ℃ static culture for 30 days, obtain mycelium and fermentation broth.
[0022] 2 Obtaining extract
[0023] Use gauze to separate the mycelium from the f...
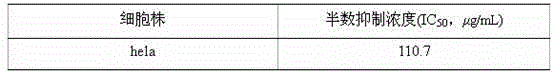
Example Embodiment
[0029] Example 2 In vitro anti-tumor activity test
[0030] 1 Experimental samples and experimental methods
[0031] Preparation of the test sample solution The test sample is the pure compound separated and refined in the above-mentioned implementation 1. Accurately weigh an appropriate amount of sample and prepare a solution of the required concentration with methanol for activity measurement.
[0032] Tumor cell lines were used for the subculture of cell lines and cells. Tumor cells were subcultured in DMEM medium containing 10% FBS at 37°C in an incubator filled with 5% carbon dioxide.
[0033] Cell proliferation inhibitory activity test method
[0034] Tetrazolium salt (MTT) method Take tumor cells in logarithmic growth phase and adjust the cell density to 2×10 per ml 5 Cells were seeded in a 96-well cell culture plate at 200 microliters per well, and 5% CO was injected at 37℃ 2 Incubate in the incubator for 4 hours. Add 2 microliters of sample solution or blank solution to each ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap