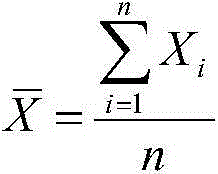Preparation method of D-dimer quality controls
A technology of dimer matrix and physique, applied in the preparation of test samples, material inspection products, biological testing, etc., can solve the problems of interference test results, clinical results, test result errors, etc., to simplify the preparation process, process Simple, quick and low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
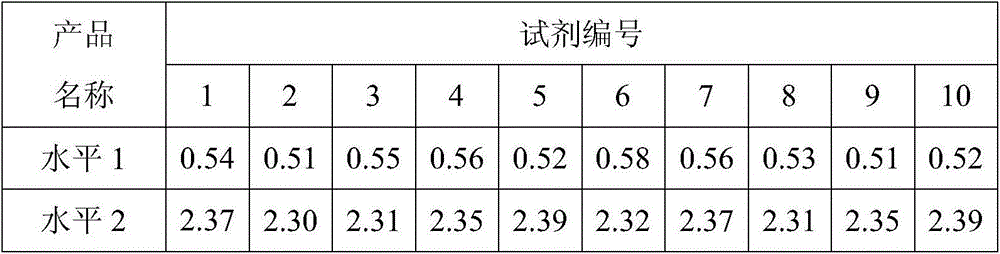
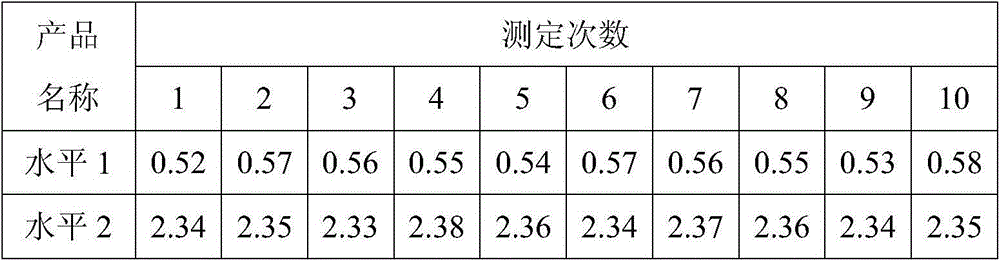
[0024] The preparation method of D-dimer quality control product, comprises the following steps:
[0025] (1) Preparation of enzyme complex solution: calcium chloride, thrombin, and urinary plasminogen activator are added to the buffer, so that their concentrations are respectively 0.03mol / L, 5U / ml, and 20mU / ml, and the obtained Incubate the enzyme complex solution at 37°C for 10-15 minutes;
[0026] (2) Preparation of fibrin clot: Incubate the mixed plasma of several people at 37°C for 5-10 minutes, mix it with the incubated enzyme complex solution, mix it upside down, and incubate at 37°C to form a fibrin clot piece;
[0027] (3) Preparation of D-dimer mother solution: wash the fibrin clot with buffer, squeeze out the plasma in the fibrin clot, then resuspend the fibrin clot with buffer, and incubate at 37°C for 1 hour , to dissolve the fibrin clot, add 2ug / ml aprotinin, centrifuge at 10000g, 4°C for 15 minutes, collect the supernatant, and obtain the D-dimer mother liquor...
Embodiment 2
[0032] The preparation method of D-dimer quality control product, comprises the following steps:
[0033] (1) Preparation of enzyme compound solution: calcium chloride, thrombin, and tissue plasminogen activator are added to the buffer solution so that their concentrations are respectively 0.01mol / L, 3U / ml, and 10mU / ml to obtain The enzyme complex solution was incubated at 37°C for 10-15 minutes;
[0034] (2) Preparation of fibrin clot: Incubate the mixed plasma of several people at 37°C for 5-10 minutes, mix with the incubated enzyme complex solution, invert and mix well, and incubate at 37°C to form a fibrin clot ;
[0035] (3) Preparation of D-dimer mother solution: wash the fibrin clot with buffer, squeeze out the plasma in the fibrin clot, then resuspend the fibrin clot with buffer, and incubate at 37°C for 0.5 hours , to dissolve the fibrin clot, add 10ug / ml aprotinin, centrifuge at 10000g, 4°C for 15 minutes, collect the supernatant, and obtain the D-dimer mother liqu...
Embodiment 3
[0040] The preparation method of D-dimer quality control product, comprises the following steps:
[0041] (1) Preparation of enzyme complex solution: calcium chloride, thrombin, and streptokinase are added to the buffer solution to make their concentrations respectively 0.05mol / L, 1 U / ml, and 5mU / ml. Incubate at 37°C for 10-15 minutes;
[0042] (2) Preparation of fibrin clot: Incubate the mixed plasma of normal people at 37°C for 5-10 minutes, mix with the incubated enzyme complex solution, invert and mix well, and incubate at 37°C to form a fibrin clot ;
[0043] (3) Preparation of D-dimer mother solution: wash the fibrin clot with buffer, squeeze out the plasma in the fibrin clot, then resuspend the fibrin clot with buffer, and incubate at 37°C for 2 hours , to dissolve the fibrin clot, add 10ug / ml aprotinin, centrifuge at 10000g, 4°C for 15 minutes, collect the supernatant, and obtain the D-dimer mother liquor;
[0044] (4) Preparation of D-dimer quality control product: a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com