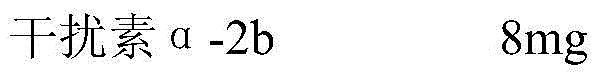Preparation and application of novel interferon alpha-2b wet tissue
A technology of interferon α, -2b, applied in skin care preparations, medical preparations containing active ingredients, cosmetic preparations, etc., can solve the problem of loss of biological activity, no interferon α-2b wet wipes, interference Loss of activity of α-2b and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, novel interferon alpha-2b wet tissue
[0044] A novel interferon α-2b wet tissue is composed of a medicinal solution and a non-woven fabric bearing the medicinal solution, wherein the medicinal solution contains:
[0045] Recombinant Human Interferon α-2b 8mg
[0046] Sodium Propylene Glycol Alginate 2mg
[0047] Purified water 90mg
[0048] The non-woven fabric adopts spunlace non-woven fabric, and the specifications meet: the ingredients are viscose and polyester, and the content of viscose fiber is between 30-60%, and the weight of the non-woven fabric used is in the range of 40-60g / m 2 .
[0049] The preparation method of this wet tissue comprises the following steps:
[0050] 1) Weighing: Weigh recombinant human interferon α-2b, propylene glycol sodium alginate and purified water according to the above dosage;
[0051] 2) Mixing: add an appropriate amount of purified water to recombinant human interferon α-2b and propylene glycol sodium alginate,...
Embodiment 2
[0054] Embodiment 2, novel interferon alpha-2b wet tissue
[0055] A novel interferon α-2b wet tissue is composed of a medicinal solution and a non-woven fabric bearing the medicinal solution, wherein the medicinal solution contains:
[0056] Recombinant Human Interferon α-2b 8mg
[0057] Sodium alginate 2mg
[0058] Purified water 90mg
[0059] The non-woven fabric is made of all-cotton non-woven fabric, and the specifications meet: the composition is natural cotton fiber, and the weight of the non-woven fabric used is in the range of 40-60g / m 2 .
[0060] The preparation method of this wet tissue comprises the following steps:
[0061] 1) Weighing: Weigh recombinant human interferon α-2b, sodium alginate and purified water in proportion;
[0062] 2) Mixing: add an appropriate amount of purified water to recombinant human interferon α-2b and sodium alginate, stir until dissolved, then add the remaining purified water under stirring, and stir until completely dissolved to...
Embodiment 3
[0065] Embodiment 3, novel interferon alpha-2b wet tissue
[0066] A novel interferon α-2b wet tissue is composed of a medicinal solution and a non-woven fabric bearing the medicinal solution, wherein the medicinal solution contains:
[0067] Interferon alpha-2b 10mg
[0068] Propylene Glycol Potassium Alginate 1mg
[0069] Purified water 89mg
[0070] The non-woven fabric is made of full viscose non-woven fabric, and the specifications meet: the composition is viscose fiber, and the weight of the non-woven fabric used is in the range of 40-60g / m 2 .
[0071] The preparation method of this wet tissue comprises the following steps:
[0072] 1) Weighing: Weigh interferon α-2b, propylene glycol potassium alginate and purified water in proportion;
[0073] 2) Mixing: add an appropriate amount of purified water to interferon α-2b and propylene glycol potassium alginate, stir until dissolved, then add the remaining purified water while stirring, and stir until completely dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com