A method for activating and preparing human thrombin from human coagulation factor ⅸ chromatography waste liquid
A technology of human coagulation factor and human thrombin, which is applied in the field of activation and preparation of human thrombin, can solve the problems of unmanned thrombin, potential safety hazards, and inability to prepare at the same time, achieving simple process, reduced incidence, and clinical virus safety guarantee Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In this example, the chromatographic flow-through was used as the chromatographic waste liquid, and the crude PCC and the finished product of human coagulation factor VIII activated human thrombin.
[0040] Step 1: Adjust the pH of 2L of human plasma to 7.0, add 3g of DEAE Sephadex A-50 gel at a ratio of 1.5g / L, and absorb for 60 minutes; with 20mmol / L sodium citrate and 200mmol / L sodium chloride solution, Wash miscellaneous protein 3 times at pH 7.0, elute 3 times with 20mmol / L sodium citrate, 1mol / L sodium chloride solution, pH 7.0, desalt the eluate by ultrafiltration until the conductivity is 20mS / cm, volume 90ml , which is the crude product of PCC, and the detected prothrombin activity is 15.1IU / ml.
[0041] The second step: Add the crude PCC obtained in the first step to the pre-prepared Tween-80 / TnBP at a ratio of 1:9, incubate at 24±1°C for 360 minutes, and inactivate the lipid-enveloped virus, and the volume after inactivation is 100ml.
[0042] The third step: ...
Embodiment 2
[0049] In this example, the chromatographic washing liquid was used as the chromatographic waste liquid, and the crude product of PCC and the finished product of human coagulation factor VIII activated human thrombin.
[0050] The first step: the eluate is desalted by ultrafiltration to a conductivity of 10mS / cm.
[0051] The third step: Divide the PCC crude product obtained in the second step after virus inactivation according to 1:19 (V / V) into two parts (1 / 20 is liquid a: 5ml, 19 / 20 is liquid b: 95ml), of which Liquid b was adsorbed by Heparin Sepharose FF gel, first washed with 0.1mol / L sodium citrate, 0.15mol / L sodium chloride solution pH7.0, and then washed with 0.1mol / L sodium citrate, 0.5mol / L The human coagulation factor IX product was obtained by eluting with sodium chloride solution at pH 7.0, and the chromatographic washing liquid was chromatographic waste liquid.
[0052] All the other conditions are with embodiment 1.
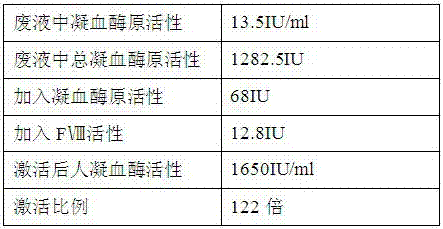
[0053] The ratio of human thrombin activi...
Embodiment 3
[0055] In this example, the chromatographic flow-through was used as the chromatographic waste liquid, and the finished product of PCC and the finished product of human coagulation factor VIII were used to activate human thrombin.
[0056] The third step: after the virus inactivation obtained in the second step, the PCC crude product was adsorbed on Heparin Sepharose FF gel, and eluted with 0.1mol / L sodium citrate and 0.5mol / L sodium chloride solution pH7.0 to obtain human Coagulation factor IX products. The chromatographic flow-through is the chromatographic waste liquid.
[0057] Step 4: Add 5% PCC (PCC finished product, 17IU / ml, 4ml), 1% human coagulation factor VIII (FVIII finished product, 22IU / ml, 580μl), 25mmol / L to the chromatographic waste liquid obtained in the third step CaCl 2 , incubated at 20°C for 3 hours, and then activated. The ratio of human thrombin activity / human prothrombin activity before and after activation was 114 times.
[0058] All the other condi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com